News Story
New Barrier Gel Will Improve Analysis of Blood Samples
Mechanism for gelation of blood by hm-chitosan. On the left the polymer is shown schematically with its hydrophilic backbone in blue and the grafted benzyl-octadecyl hydrophobes in purple. When added to liquid blood, the components assemble into a three-dimensional network (gel), as shown on the right. This is driven by insertion of hydrophobes into blood cell membranes (as depicted in the top inset); thereby the polymer chains connect (bridge) the cells into a self-supporting network.
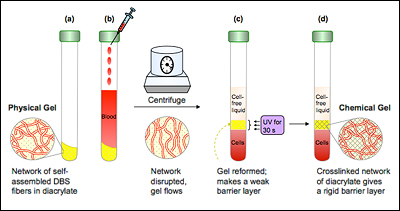
There is a small amount of gel at the bottom of each slender tube, and it has a very important job to do: When blood samples are put into a centrifuge and spun to separate the heavier cells from the lighter underlying liquid (called plasma or serum), the gel, which has a density between them, ends up in the middle, creating a barrier. Since lab tests typically require only the serum, this allows doctors and technicians to easily draw it off for analysis. But the barrier is soft, and can leak during storage or transportation, contaminating the sample.
Frustrated by the problem and armed with an idea for a solution, Dr. Jane Emerson, a clinical pathologist from the University of Southern California, sought out Clark School Department of Chemical and Biomolecular Engineering (ChBE) associate professor Srinivasa Raghavan, whose Complex Fluids and Nanomaterials Group specializes in soft "smart" materials. Together with ChBE graduate students Kunshan Sun (Ph.D. '09) and Hyuntaek Oh, the team developed an innovative new separator gel capable of forming a permanent, solid barrier between cells and serum.
"Dr. Emerson approached us with some specifications and a great technical challenge," Raghavan explains. "Using our knowledge of polymer science, colloid/nano science, and self-assembly, we designed a material that initially has the consistency of ketchup or paint, but after exposure to ultraviolet light converts to a consistency of stiff rubber. The initial state is essential for it to flow between the layers of blood—the cells and serum—during centrifugation and form a soft barrier, like existing separator gels. The secondary state ensures that it remains permanent and leakproof."
The “ketchup-like” consistency was achieved by the self-assembly of a sorbitol-based gelator (a substance capable of forming a gel) into a nano-fibrous network within a UV curable oligomer (a material whose molecules consist of a few monomer units, as opposed to being made of long polymer chains), which can be hardened by exposure to ultraviolet light. The bonds within the nano-fiber are weak, non-covalent bonds that are susceptible to shear (the force applied by the centrifuge), allowing it to migrate to the middle of the phial during the separation process. The “rubber-like” consistency was achieved by curing (hardening) the oligomer with UV light, which solidifies the separator gel into a cross-linked polymer network held together by strong covalent bonds.
The new material had to meet stringent requirements to ensure it would perform physically while not interfering with blood components, clotting, or clinical laboratory assay methods.
According to Raghavan, he and Emerson have filed a patent on the separator gel. Emerson is currently working with Polymer Solutions, Inc. an independent laboratory, to develop a commercial version of the product. The gel also has potential industrial applications in which the separation of materials within the same container is required.
For additional media coverage of this research, see:
Chemistry World: "Blood barrier gel aids medical analysis" »
Polymer Solutions Newsblog: "Barrier Gel Advances Blood Analysis" »
"A new method for centrifugal separation of blood components: Creating a rigid barrier between density-stratified layers using a UV-curable thixotropic gel." Kunshan Sun , Hyuntaek Oh , Jane F. Emerson and Srinivasa R. Raghavan J. Mater. Chem., 2012, 22, 2378-2382. Abstract »
For More Information:
Visit the Complex Fluids and Nanomaterials Group web site »
Published April 4, 2012