Press Release
New electrolyte bolsters rechargeable battery design
Researchers overcome challenges in magnesium, calcium battery development with new design principle.
FOR IMMEDIATE RELEASE October 8, 2021
CONTACT:
Katie Holland
301 405 0379
khollan3@umd.edu
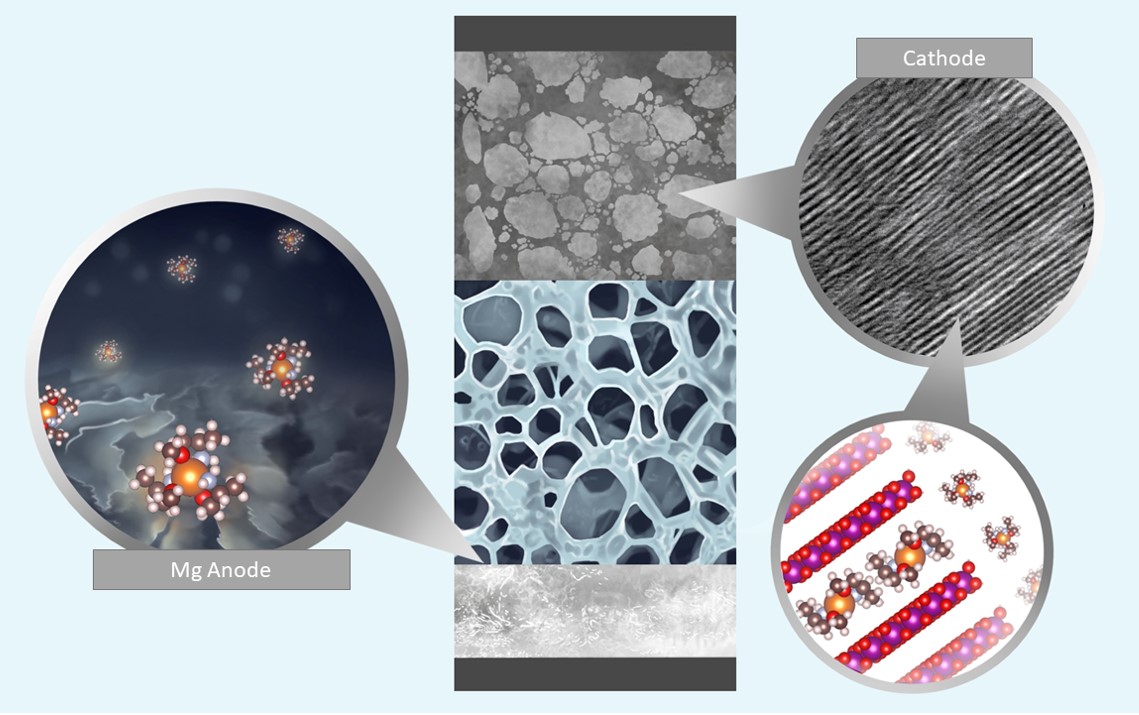
Battery electrolytes using amine-based chelants solvating divalent cations demonstrated stable and highly reversible plating/stripping of Mg metal with the scanning electron microscopy (SEM) images of the cycled Mg anode shown on a left together with a representative solvation shell of the Mg2+ cation. Credit: Nina Borodin, Singyuk Hou, Xiao Ji for UMD.
The energy contained within lithium-ion batteries has the potential to reshape the technology of the future battlefield, creating a worldwide demand for key lithium-ion battery materials such as lithium, cobalt and nickel, posing a supply problem for future production.
Researchers at the University of Maryland (UMD) and the U.S. Army Combat Capabilities Development Command, known as DEVCOM, Army Research Laboratory designed a breakthrough battery electrolyte that may open new possibilities for promising alternatives, such as rechargeable magnesium metal batteries. A detailed study about this novel battery technology was published in Science on October 8.
“Magnesium is substantially more abundant than lithium, which should meet the needs of the ever-growing battery market,” said Oleg Borodin, Army computational chemist. “The low redox potential and large capacity of magnesium metal anode could also potentially provide an energy density comparable or even greater than that of lithium-ion batteries when paired with high voltage oxide cathodes.”
Borodin also explained that compared to lithium, magnesium forms less dendrites, which experts pinpoint as the main cause of safety concerns in lithium-ion batteries.
Despite these advantages, magnesium metal batteries still face many issues that hinder their development. One major problem pertains to magnesium’s strong reaction to conventional electrolytes during battery operation – both electrodes must be compatible with the electrolyte in order for the battery to attain a sufficient energy density.
As an anode material, magnesium has a tendency to corrode the electrolyte and create a thick coating around the anode.
While similar coatings in lithium-ion batteries enable the diffusion of lithium ions and protect the electrolyte from further decomposition, this coating instead blocks the magnesium plating and inhibits the necessary electrochemical reactions from occurring.
In an effort to address this problem, a UMD research team led by Chunsheng Wang, professor of chemical engineering and director of the Center for Research in Extreme Batteries, developed a novel design strategy that incorporated a new class of solvents.
To their surprise, the electrolyte design not only prevented the corrosion process, but it also dramatically increased the reaction kinetics of both the anode and the cathode, boosting the battery’s overall performance.
“Previous electrolytes could plate magnesium, but they had a lot of drawbacks,” Wang said. “This research solved this problem with a new electrolyte that allowed the plating of magnesium metal but also the usage of higher voltage cathodes. It’s the first time a magnesium battery theoretically reached a similar energy density as a lithium-ion battery.”
The researchers also found they could apply the same design principle to other materials that fall under the category of divalent metals, not just magnesium.
In their experiment, the new electrolyte design strategy allowed the researchers to circumvent commonly observed challenges for both rechargeable magnesium metal batteries and rechargeable calcium metal batteries.
“The test with calcium demonstrates that this electrolyte design could be extended to other types of multivalent batteries that have low potential and broaden the choice of electrode materials in general,” said Singyuk Hou, UMD graduate student and co-author of the study. “People are especially interested in calcium because the potential of a calcium anode is even lower than that of a magnesium anode, and calcium is also very abundant in the earth’s crust.”
Army researchers mainly supported the study through density functional theory (DFT) calculations that helped the team understand why the new electrolyte led to these improvements and guide search for new electrolytes.
“The Army helped us understand the mechanics of exactly what happened,” Wang said. “We have knowledge about the phenomenon, but the calculations they provided played an important role in demonstrating this technology and what we should do next to make it better.”
According to Wang, the team plans to optimize the electrolyte and then upscale the concept into a large-scale power cell.
Borodin explained that this new design strategy could signify a real breakthrough for divalent metal batteries after two decades of research fraught with issues related to insufficient energy density caused by electrode-electrolyte incompatibility. This advancement could change how the Army supplies power to soldiers on the battlefield in the future.
Follow this link to ready the study in Science.
About the A. James Clark School of Engineering
The University of Maryland’s A. James Clark School of Engineering is a premier program, ranked among the top 20 in the world. Located just a few miles from Washington, D.C., the Clark School is at the center of a constellation of high-tech companies and federal laboratories, offering students and faculty access to unique professional opportunities.
Our broad spectrum of academic programs, including the world’s only accredited undergraduate fire protection engineering program, is complemented by a vibrant entrepreneurial ecosystem, early hands-on educational experiences, and participation in national and international competitions.
The Clark School is leading research advancements in aerospace, bioengineering, robotics, nanotechnology, disaster resilience, energy and sustainability, and cybersecurity. From the universal product code to satellite radio, SMS text messaging to the implantable insulin pump, our students, faculty, and alumni are engineering life-changing innovations for millions. Learn more at www.eng.umd.edu.