Press Release
Hybrid Electrolyte Bridges Gap Between Aqueous and Non-aqueous Battery Technology
Study conducted by UMD Professor Chunsheng Wang and U.S. ARL Scientist Kang Xu's research groups, published in Joule.
FOR IMMEDIATE RELEASE March 12, 2018
CONTACT:
Katie Doyle
301 405 0379
khollan3@umd.edu
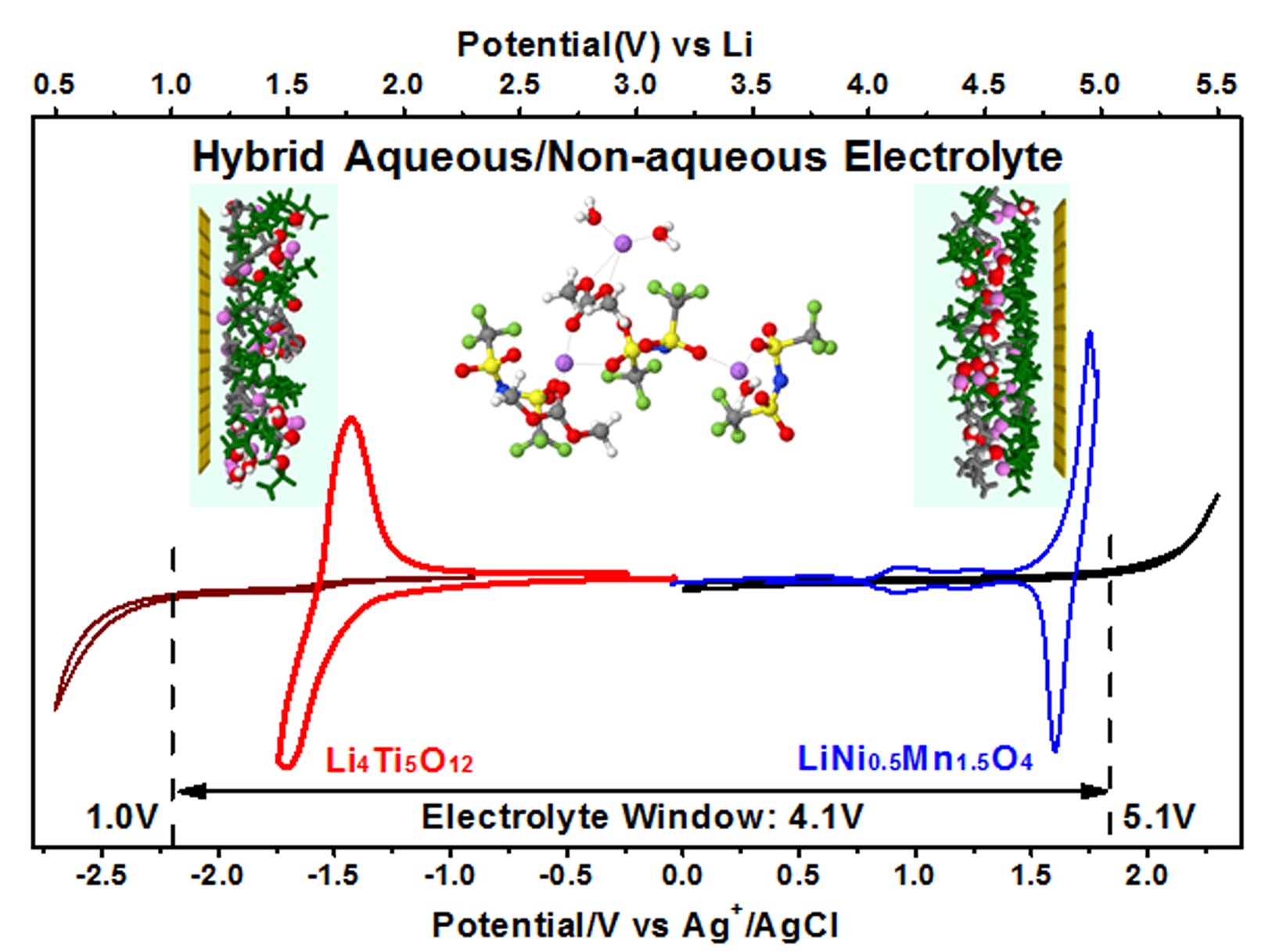
Hybrid aqueous/non-aqueous electrolyte (HANE) inherits merits from both aqueous (non-flammability) and non-aqueous (high electrochemical stability) systems. Performance comparable with state-of-the-art Li-ion batteries.
College Park -- Engineers at the University of Maryland (UMD) Department of Chemical and Biomolecular Engineering (ChBE) and U.S. Army Research Lab (ARL) – led by Professor Chunsheng Wang (UMD) and Dr. Kang Xu (ARL) – have developed an aqueous/non-aqueous battery chemistry, which blends the strongest solvents from both water-based and non-water-based designs, to create a hybrid electrolyte that is highly efficient, cost-effective and eco-friendly with a high energy density. This hybrid aqueous/non-aqueous electrolyte, or HANE, is non-toxic and non-flammable, relaxing the safety concerns associated with the conventional Li-ion battery.
This study, entitled, “Hybrid Aqueous/Non-aqueous Electrolyte for Safe and High-Energy Li-ion Batteries,” published online today in Joule. Dr. Fei Wang, a UMD/ARL joint post-doctoral researcher, served as the paper’s first author.
In conducting this research, “we were motivated by the fact that the solid electrolyte interphase derived from the decomposition of the TFSI anion could extend the stability window of the water, which was reported by our group in Science,” Fei Wang stated. “To further extend the window, we introduced an organic component that not only rearranges the interfacial structure near the inner-Helmholtz layer, but that could also decompose to form an additional SEI component. Based on the composite interphases, the thermodynamic stability window of the water (1.23 volts) was expanded to a 4.1 volts, which could support more electrode material choices.”
A unique feature of this design is that it can be manufactured in open-air.
“Conventional Li-ion batteries, by comparison, require a rigorous moisture-free environment – a dry-room is a must,” Professor Wang clarified. “The resultant batteries have to be hermetically-sealed making them rather rigid, which essentially eliminates any flexibility.” HANE renders batteries unprecedented freedom from such rigidity. “We imagine that in the future, batteries can be designed to work in open atmosphere, or printed into any shape required by individual devices,” said Professor Wang.
This new technology, having numerous applications, still has one main obstacle: “the electrolyte needs several (charge/discharge) cycles to form a stable SEI on the anode surface, which sacrifices some energy density,” Fei Wang explained.
“In the future, we will do some pretreatment on the anode surface to shorten or remove the formation process," Dr. Kang added. "It’s still a work in progress, but we hope to commercialize this product in the near future.”
For additional information:
Wang et al. Hybrid Aqueous/Non-aqueous Electrolyte for Safe and High-Energy Li-ion Batteries, Joule (12 March, 2018), https://doi.org/10.1016/j.joule.2018.02.011
-- end
About the A. James Clark School of Engineering
The University of Maryland’s A. James Clark School of Engineering is a premier program, ranked among the top 20 in the world. Located just a few miles from Washington, D.C., the Clark School is at the center of a constellation of high-tech companies and federal laboratories, offering students and faculty access to unique professional opportunities.
Our broad spectrum of academic programs, including the world’s only accredited undergraduate fire protection engineering program, is complemented by a vibrant entrepreneurial ecosystem, early hands-on educational experiences, and participation in national and international competitions.
The Clark School is leading research advancements in aerospace, bioengineering, robotics, nanotechnology, disaster resilience, energy and sustainability, and cybersecurity. From the universal product code to satellite radio, SMS text messaging to the implantable insulin pump, our students, faculty, and alumni are engineering life-changing innovations for millions. Learn more at www.eng.umd.edu.