News Story
Gaging the Long-Term Performance of Nuclear Reactor Cooling Pipes
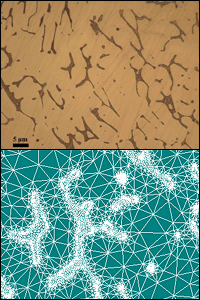
Top image: The microstructure of the stainless steel CF3, with its two phases clearly distinguishable. Bottom image: An elarged view of the digitized microstructure of the same sample, with a finite element modeling (FEM) mesh applied.
The U.S. Department of Energy (DOE) has awarded Department of Materials Science and Engineering (MSE) graduate student Samuel Schwarm a three-year, $155,000 Nuclear Energy University Programs (NEUP) Fellowship. The award, which also includes tuition, a travel grant, and a ten-week internship with a DOE-affiliated institution, will support Schwarm’s long-term study of the materials used in the cooling systems of commercial nuclear reactors.
In the United States, the Nuclear Regulatory Commission licenses commercial nuclear reactors to operate for 40 years, with the possibility of a 20-year extension. As the majority of the country’s reactors approach or have surpassed that age, their components have been evaluated for current and continued safety and stability. This includes two types of corrosion-resistant cast duplex stainless steel, known as CF3 and CF8, which are used to manufacture the water pipes in the reactors’ cooling systems. Can they continue to operate as designed for another 20-30 years, or should they be replaced?
The DOE has turned to Schwarm and the members of MSE professor Sreeramamurthy Ankem’s research group for answers.
The industrial-grade stainless steels, Schwarm explains, are what are known as multiphase materials. Each is an alloy of iron, nickel and chromium, but contain two or more molecular configurations, or phases, within a bulk sample, such as a pipe. Combined, the two phases’ unique characteristics create a superior material. Over time and at varying high operating temperatures, however, atoms migrate (diffuse) and the composition of each phase begins to change. This ultimately has an effect on their properties.
Most in-depth studies of reactor cooling pipes made of CF3 and CF8 last occurred in the 1990s, when many of them were about 20 years old. Phase changes were noted, but determined to have no serious effect on the pipes’ ability to perform as designed for the balance of their projected 40-year lifespans
“The previous work shed light on the changing mechanical properties and similar aspects for operation,” says Schwarm. “We’re trying to take things a step further by investigating why and how this property evolution is occurring. The DOE wants to see if we can use these pipes longer, up to 70 years.”
To find out, Schwarm and his colleagues have undertaken a three-year study in which samples of CF3 and CF8 are aged at the two operating temperatures they would experience in a reactor’s cooling system – 280 and 320 degrees Celsius (536 and 608 degrees Fahrenheit) – 24 hours a day, seven days a week, 365 days a year. Schwarm is also performing accelerated aging on samples subjected to even higher temperatures. The structural changes to the samples are the same, but the pace at which they occur is sped up; a year at 400° C is about the same as 2.3 years at 280° C.
Although the pipes are filled with water when installed in nuclear power plants, Schwarm explains that the lack of water in his tests has a minimal, if any, effect on his results. “One of the reasons they chose this material is because that as a stainless steel, it doesn’t react with water the way regular steel does. It oxidizes to a small degree, but not to the point of causing dramatic changes in its properties.”
Every six months, samples are removed from their ovens and quenched (rapidly cooled), which “freezes” their molecular structure. Changes in phase and mechanical properties are observed using electron microscopy, impact testing, and tensile testing.
The physical experiments establish how much force is required to break and deform the metal. Results show that over time, it takes more stress to deform it, but that it also fractures more easily after deformation. “The samples are strengthening but becoming more brittle at the higher temperatures,” Schwarm explains.
Microscopy reveals what has happened to the steel’s microstructure. Over time, both of the steel’s two original phases change. One of them splits into two sub-phases with distinct compositions. All three phases are dominated by iron, but one has a relatively large proportion of nickel and another has a relatively large proportion of chromium. The third contains the most iron.
After gathering the physical data and microscopy images, Schwarm uses them to create new computer simulations of the steel using a technique called finite element modeling (FEM). The model is mapped to a digital mesh, allowing him to predict what will happen to the bulk structure of the material when force is applied to various points. Because the model has been created from data and observations of physical experiments, its predictions are much more accurate.
The modeling, Schwarm says, has applications beyond the current project that he hopes will contribute to the field.
“Very little FEM modeling of two phase and multiphase materials at the microstructure level has been done,” he explains. “Most research in this area has relied on approximate models. The technique we’re developing can be applied to other materials, not just these steels.”
Schwarm, who earned his B.S. at the University of Alabama Tuscaloosa, knew he wanted to pursue a Ph.D., but initially wasn’t sure what he was looking for in a graduate program. Having majored in metallurgy and performed undergraduate research in magnetic materials, he began to look into MSE at the University of Maryland, contacting faculty members who specialize in both areas. He ultimately scheduled a visit with Ankem.
“Talking to Dr. Ankem, and working with him…it was just a good fit and we got along very well,” he says. “[His work] just sounded like something I wanted to do and something my background prepared me for. So far it’s worked out even better than I expected. I am very excited to have been offered this opportunity to represent the university.”
Published June 2, 2015









