News Story
UMD Research Group Creates Cheap, Membrane-Free Chlorine Battery
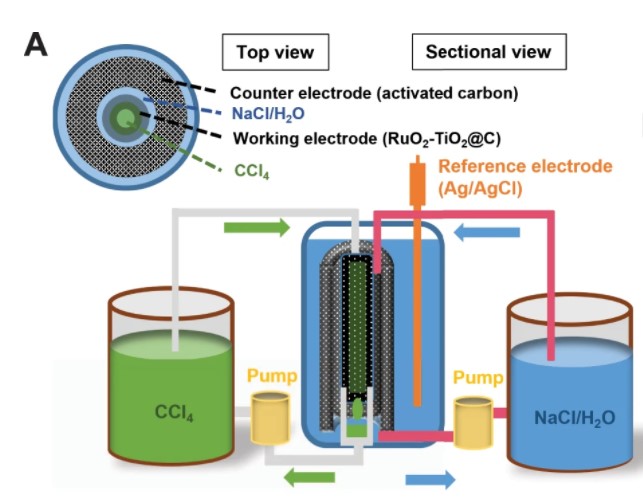
Incorporating large-scale renewable energy, such as solar or wind power, into power grids is essential for reducing greenhouse emissions. Redox flow batteries (RFB) – electrochemical energy storage devices that convert chemical energy into electrical energy via reversible oxidation and reduction of fluids – provide affordable and scalable solutions for stationary energy storage. However, most of the current RFB chemistries are based on expensive transition metal ions, or synthetic organics.
To remedy the situation, a research group in the University of Maryland (UMD) Department of Chemical and Biomolecular Engineering (ChBE) led by Chunsheng Wang has created a reversible chlorine redox flow (Cl2/Cl-) battery conceptualized by the chlorine production in chloro-alkali industry. In particular, the chlorine is produced by oxidizing the chloride ions in the aqueous sodium chloride solution and stored in the water-immiscible organic flow. This design has enabled highly reversible energy storage and the removal of the costly ion-permeable membrane used in most redox flow batteries.
“What we’ve done, essentially, is made the chloride to chlorine conversion reversible, allowing us to use this extremely accessible resource, sodium chloride, for potential large-scale grid-level energy storage,” said Singyuk Hou, a ChBE Graduate Student and first author on the study. “This was achieved by implementing a multi-phase flow, meaning, one acts as reactor while the other acts as the collector.”
Wang added: “There will be a significant mismatch between energy-demanding time and harvesting time when using solar and wind power if they’re integrated into the electrification grid. We cannot ask people to use electricity just on a sunny day. Redox flow batteries can provide a simple solution to this problem by storing the energy for future use, like an energy bank.”
This chlorine flow battery, which is highly scalable, provides a safe, reliable energy storage alternative at an affordable cost. Moreover, the membrane-free design enables both anionic and cationic charge carriers, thus expanding the chemical space for redox-active materials to be explored for RFBs in the future.
For additional information:
Hou, S., Wang, C. et al. (11March2022). High energy and low-cost membrane-free chlorine flow battery, Nature Communications. DOI: 10.1038/s41467-022-28880-x
Published March 23, 2022









