News Story
New Storage for Hydrogen Fuel
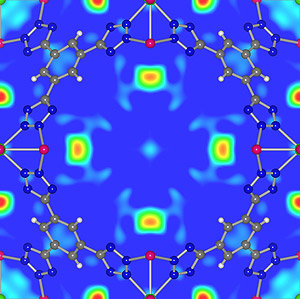
The arrangement of hydrogen molecules absorbed by a metal/organic "framework" material (MOF) developed by the team of Clark School and NIST researchers.
Auto manufacturers are investing billions of dollars to develop hydrogen-powered, fuel-cell based cars and trucks, which require major advances in the production and storage of hydrogen to reduce CO2 in the atmosphere and allow a transition away from the use of the world’s diminishing oil supplies. One of the major challenges in the quest to develop hydrogen fueled vehicles is the creation of lightweight materials that will absorb and release hydrogen at moderate temperatures and pressures.
The behavior of hydrogen can be difficult to study. Devices that use x-rays detect and identify atoms by the electrons that orbit an atom's nucleus. Hydrogen atoms have only one electron each, meaning there is far less for an x-ray to "see". Researchers on the hydrogen storage project are instead making use of powerful neutron-based instruments at the NCNR, developed jointly by Maryland and NIST, to precisely measure the bonding and movement of hydrogen bound in the storage materials. Like electrons, neutrons are also subatomic particles. When neutron beams are sent into a sample, they scatter from the nuclei of the atoms instead of their electrons. The way in which the neutrons scatter, including their direction and energy before and after hitting the sample, tells scientists where the hydrogen is and what it is doing. Using the neutron scattering technique, hydrogen is 10 to 30 times easier to see than other atoms making it a uniquely sensitive probe of hydrogen.
The Maryland/NIST team has successfully measured key properties of the storage materials that both they and collaborators from across the U.S. have developed. In one example, they have measured the amount, location and motion of hydrogen found in new metal/organic "framework" materials (MOFs) tailored to provide nanosized pores that can absorb and release large amounts of hydrogen. This information provides invaluable insights into the binding of absorbed gas, and helps guide the development of better materials. (The arrangement of hydrogen molecules absorbed by one of these MOFs is shown above.) The team is also finding new paths to the creation of lightweight metal hydrides that absorb more hydrogen and release it at temperatures required for vehicle operation.
This work is one of many research efforts carried out under the Maryland/NIST cooperative program on Neutron Measurements and Science, managed by MSE Professor and Chair Robert M. Briber. The equipment used is currently the best collection of such instruments in the United States. The Maryland/NIST hydrogen storage research has been presented in a number of invited lectures, including the national meetings of the American Physical Society, Materials Research Society, American Chemical Society, and the American Nuclear Society. The project was also awarded the top prize for materials research at the annual Sigma Xi poster competition at NIST. The team has formed collaborations with colleagues across the U.S., including CalTech, the Jet Propulsion Laboratory, Sandia National Laboratory, the National Renewable Energy Laboratory, UC Berkeley, and the University of Pennsylvania.
For more information, see the Department of Materials Science and Engineering's research spotlight, "New Hydrogen-Storage Materials for Future Alternative-Fuel Vehicles."
Published October 8, 2007









