News Story
UMD’s DeVoe Pioneers New Approach to Liposome Production
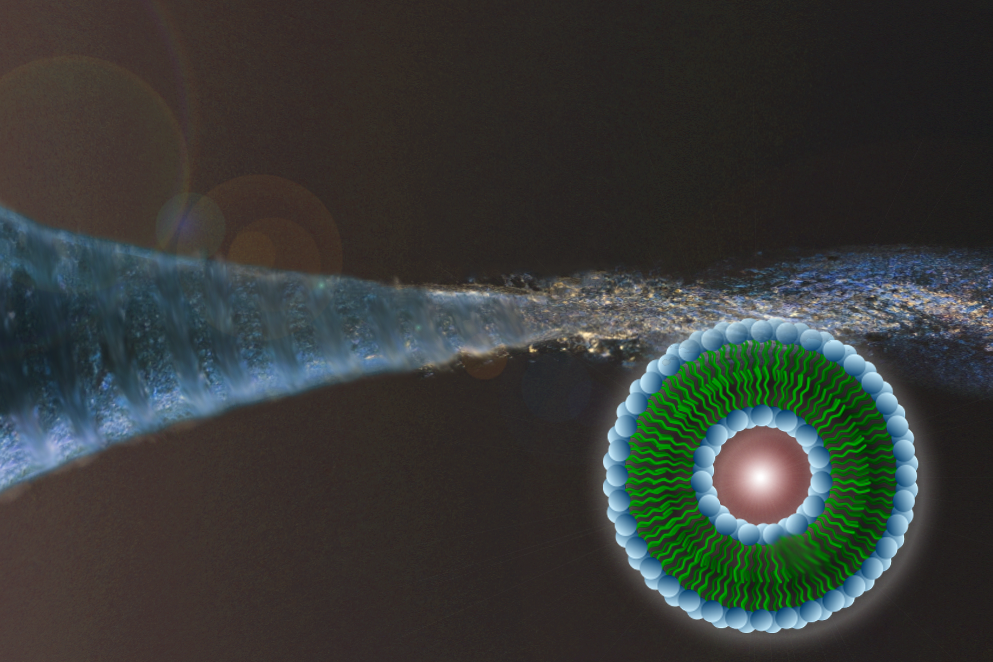
A focused microfluidic vortex enabling tunable self-assembly of size-optimized lipid nanoparticles.
Small artificial vesicles known as liposomes are used widely as nanoscale drug carriers in treatment of cancer and immune diseases, as well as in vaccine delivery and other applications, in large part because of their ability to protect medication from being degraded as it courses through a patient’s body.
As Don DeVoe, professor and associate chair of mechanical engineering at the University of Maryland (UMD) explains, “lipid-based nanoparticles are the leading nanomedicine delivery vehicles. The most successful nanomedicines to date and the vast majority of nanomedicines that are currently in development, in clinical trials, or in research labs are based on the use of lipid nanoparticles.”
Among the best-known examples: the mRNA vaccines used to protect against Covid-19. The recent pandemic also highlighted a key challenge associated with liposome-based drug delivery systems–the need to scale up and achieve high levels of throughput. “There are situations in which, out of the blue, the demand for a drug suddenly surges, with a need for enormous numbers of doses to be available,” said DeVoe, who is also a Fellow of UMD's Fischell Institute for Biomedical Devices. “The Covid-19 pandemic was one instance, and there may be others in the future.”
But the current microfluidic-based methods used to prepare lipid nanomedicines run into problems when scaled to such levels. In particular, control over the size of the lipid particles deteriorates–and that’s a major drawback, as particle size has a direct impact on drug delivery, affecting the way medication enters the bloodstream and is taken into individual cells.
DeVoe and his research team at the Maryland MEMs and Microfluidics Lab have come up with a solution, however. In a paper published recently by Nature Communications, DeVoe and co-authors Jung Yeon Han and Joseph LaFiandra detail a process that utilizes an entirely new technique known as “microfluidic vortex focusing (MVF).”
"The vortex focusing method provides exquisite control over lipid assembly kinetics, allowing the sizes of liposomes and other lipid nanoparticles to be defined by the user. While rapid microfluidic mixing has been broadly used for lipid nanoparticle preparation, such as in the production of mRNA vaccines for Covid-19, the MVF process provides a level of control over nanoparticle size that cannot be achieved by rapid mixing alone."
Don DeVoe, professor and assistant chair of mechanical engineering, University of Maryland
The technique works by creating a rapidly rotating flow in a microfluidic chamber. Lipid molecules injected into the center of the vortical flow are focused into a narrow stream while simultaneously mixing with the surrounding fluid. Through this process, diffusion and solubility of the lipids–and, as a result, particle size–can be controlled precisely.
"The vortex focusing method provides exquisite control over lipid assembly kinetics, allowing the sizes of liposomes and other lipid nanoparticles to be defined by the user,” DeVoe said. “While rapid microfluidic mixing has been broadly used for lipid nanoparticle preparation, such as in the production of mRNA vaccines for Covid-19, the MVF process provides a level of control over nanoparticle size that cannot be achieved by rapid mixing alone."
Because the microfluidic system can be operated at high flow rates with Reynolds numbers approaching the laminar limit, throughput of the technology is orders of magnitude higher than that of previous liposome synthesis techniques based solely on flow focusing, he said.
Moreover, the entire process can be scaled to very high levels of throughput without losing size control.
"A single device the size of a matchbox can produce lipid nanoparticles at a rate consistent with pilot-scale manufacturing, and multiple devices can be easily operated in parallel,” DeVoe said. “Since the platform requires very little infrastructure to run–just a pair of small pumps and a refrigerator to store reagents–we envision that it may also play a role in rapidly scaling production capacity when needed, for example in response to future pandemic threats."
Published February 1, 2023









