News Story
Innovative Chemistry Revolutionizes the Zinc-Air Battery
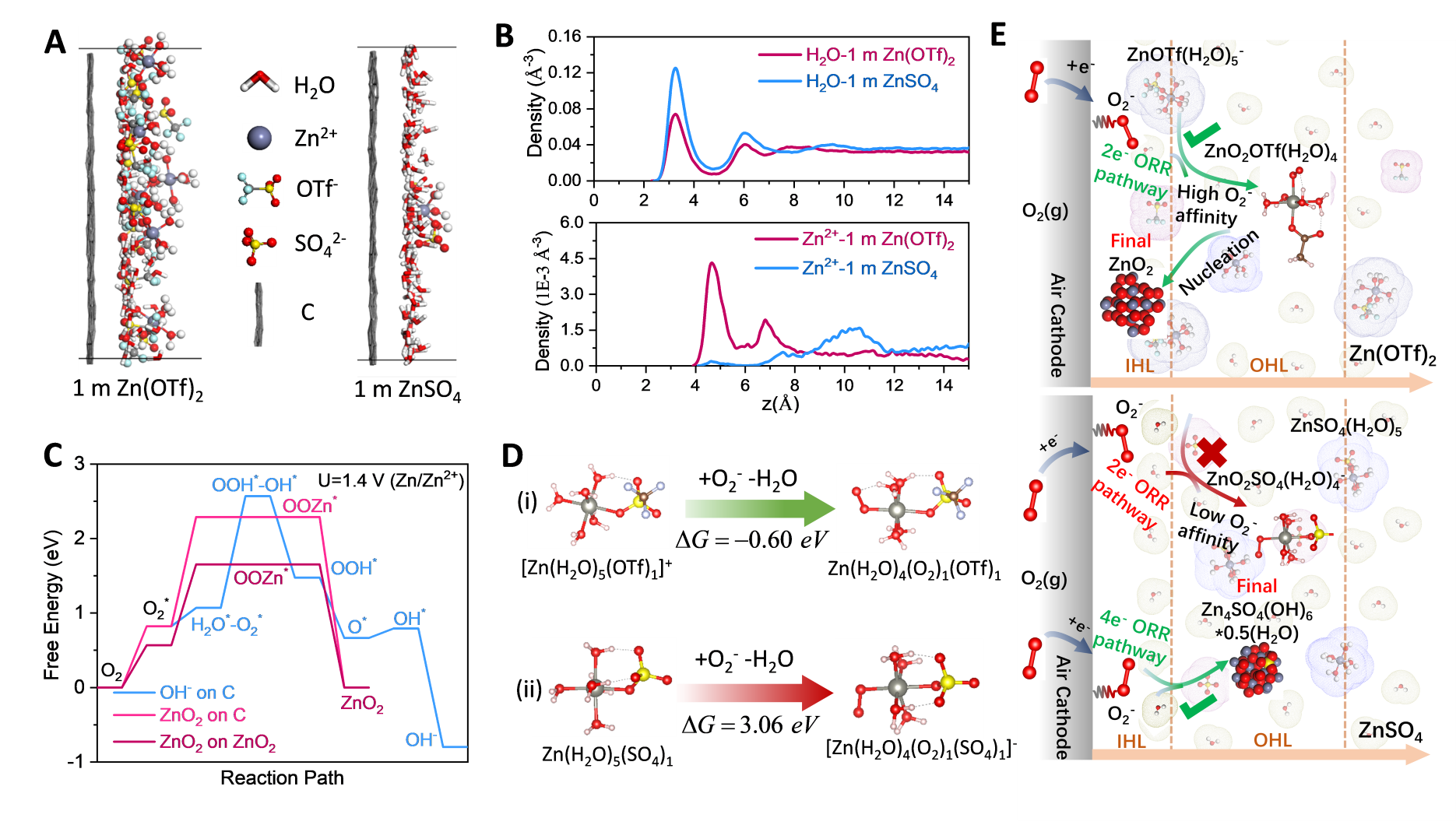
Image: Reaction processes in the inner/outer Helmholtz Layer (IHL/OHL) at the surface of air cathode in Zn(OTf)2 electrolytes.
As the potential for a global energy crisis continues to increase, so does the demand for sustainable energy resources. Research teams across the globe strive to develop high performance, eco-friendly, safe and cost-effective batteries. The zinc-air battery (ZAB) is an attractive alternative to the lithium-ion battery currently dominating the energy storage market; however, the conventional ZAB can often be unstable. Parasitic reactions, or side reactions - such as dendrite formation and air electrode failure rooted in the usage of alkaline electrolytes - often lead to battery failure and impede the development and application of ZABs.
To solve this problem, an international research team, co-led by University of Maryland (UMD) Professor, Chunsheng Wang, has developed a novel chemistry for the ZAB, which – based on a non-alkaline, water electrolyte – overcomes the parasitic reactions. Wei Sun – a former visiting Ph.D. Student in Dr. Wang’s group and now a postdoctoral researcher at the University of Münster – served as first author on the study published in Science on January 1, 2021. While working with Wang’s group, Sun focused on Zn-ion and proton co-insertion mechanism in aqueous Zn batteries
"This non-alkaline electrolyte brings a previously unknown reversible zinc peroxide (ZnO2)/O2 chemistry to the zinc-air battery," said Sun. "Compared with the conventionally strong alkaline electrolytes, this newly developed non-alkaline aqueous electrolyte has several advantages: the zinc anode is used more efficiently with a higher chemical stability and electrochemical reversibility."
"Current zinc-air batteries suffer from the slow 4 electrons oxygen (O2) redox reaction due to water involvement," said Wang. "By using a Zn-salt with a hydrophobic anion trifluoromethanesulfonate (triflate), we removed water from air cathode surface enabling the highly reversible 2e- ORR reaction on air cathode in a diluted, aqueous electrolyte; thus, the full zinc-air batteries can operate stably for 320 cycles and 1,600 hours under ambient air atmosphere.”
Although the ZAB provides a potential alternative battery technology with several advantages, this technology still requires further intensive research and optimisation.
"This zinc-air battery is designed to work in an open atmosphere, so electrolyte evaporation is inevitable," Wang said. "A water management system might be needed to ensure a long-term practical operation."
For additional information:
SunW., Wang, F., ZhangB., Zhang, M., Küpers V., Ji, X., Theile, C., Bieker, P., Xu, K., Wang, C. and Winter, M. (2021 Jan 01). "A rechargeable zinc-air battery based on zinc peroxide chemistry," Science. DOI: 10.1126/science.abb9554
Published December 31, 2020









