News Story
A Paradigm Shift in Nanochannel Research
 Nanochannels have important applications in biomedicine, sensing, and many other fields. Though engineers working in the field of nanotechnology have been fabricating these tiny, tube-like structures for years, much remains unknown about their properties and behavior.
Nanochannels have important applications in biomedicine, sensing, and many other fields. Though engineers working in the field of nanotechnology have been fabricating these tiny, tube-like structures for years, much remains unknown about their properties and behavior.
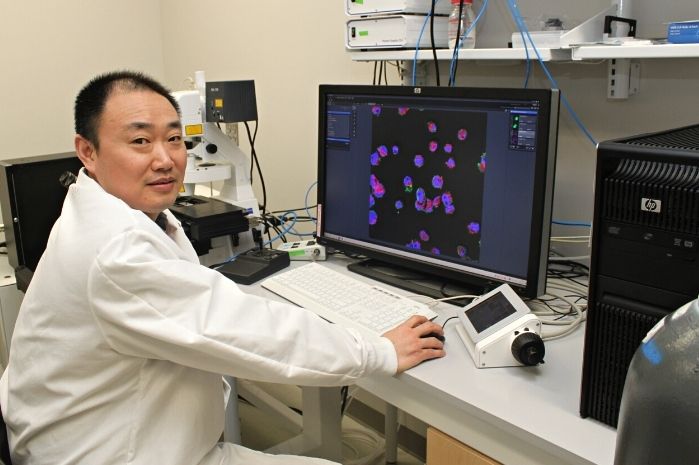
Now, University of Maryland mechanical engineering associate professor Siddhartha Das and a group of his Ph.D. students have published surprising new findings in the journal ACS Nano. Using atomic-level simulations, Das and his team were able to demonstrate that charge properties as well as charge-induced fluid flow within a functionalized nanochannel do not always behave as expected.
 “We’ve discovered a new context for nanochannels functionalized by grafting their inner walls with charged polymer molecules (also known as polyelectrolytes or PEs),” Das said, referring to the process of grafting polymers or other substances onto the nanochannel in order to cause it to function in a certain way. “The functionalization of nanochannels is not new. But we’ve come up with a paradigm shift in terms of understanding the behavior and properties of such systems in the context of their charge properties and their ability to regulate fluid flow.”
“We’ve discovered a new context for nanochannels functionalized by grafting their inner walls with charged polymer molecules (also known as polyelectrolytes or PEs),” Das said, referring to the process of grafting polymers or other substances onto the nanochannel in order to cause it to function in a certain way. “The functionalization of nanochannels is not new. But we’ve come up with a paradigm shift in terms of understanding the behavior and properties of such systems in the context of their charge properties and their ability to regulate fluid flow.”
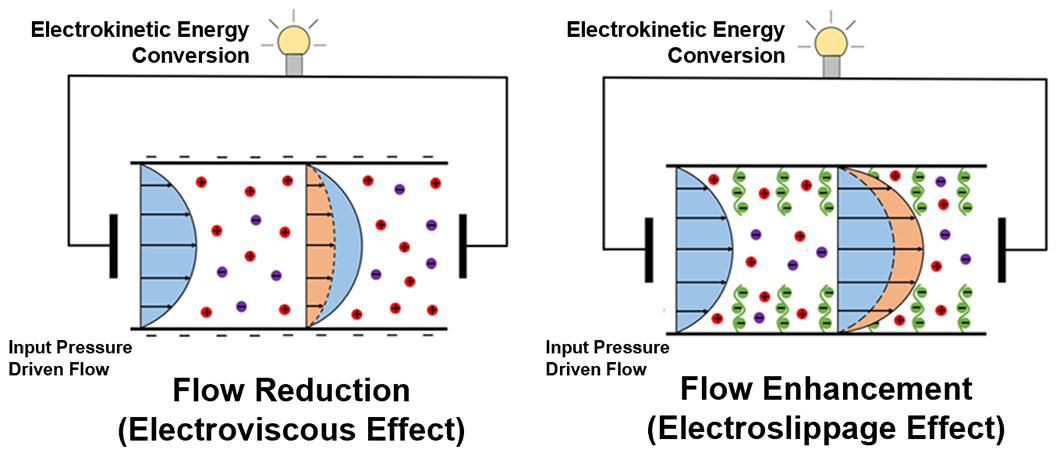
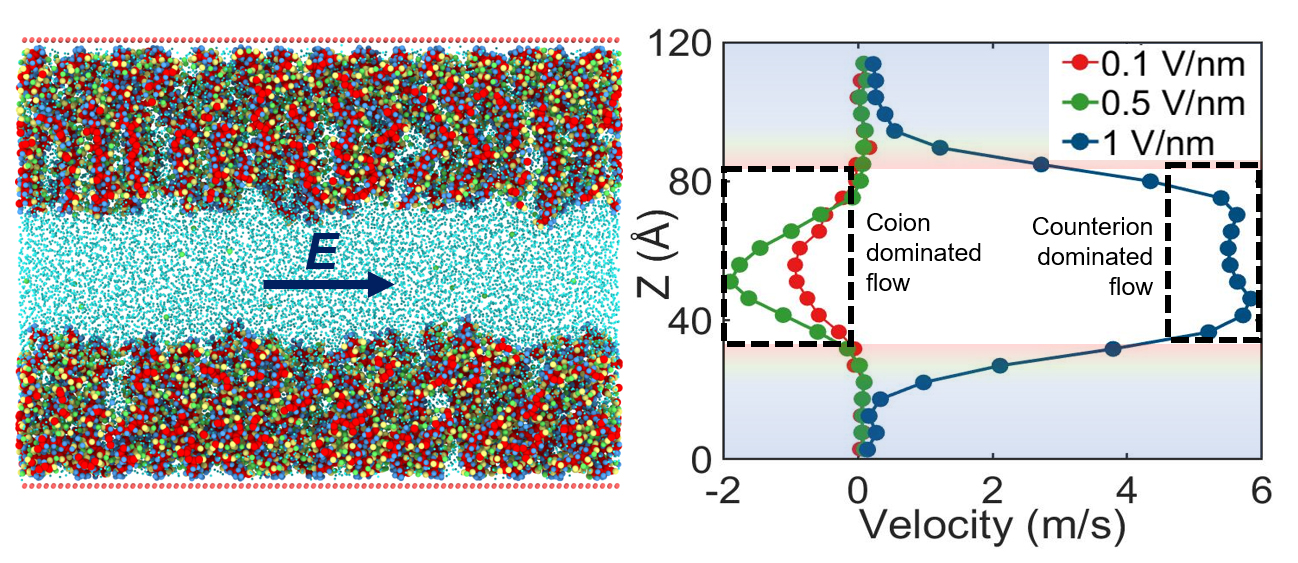
The team then investigated how this overscreening affects the external electric field driven flow (known as the electroosmotic or EOS flow) within the nanochannel. They found, surprisingly, that in such situations the flow is driven by ions having the same charge as the Pes grafted onto the channel walls; thus, a negatively charged polymer creates a net positive field in its vicinity, but the flow is driven by the negative ions.
“We call this ‘co-ion driven electro-osmosis,’ and our paper marks the first time this phenomenon has been identified,” Das said.
“The functionalization of nanochannels is not new. But we’ve come up with a paradigm shift in terms of understanding the behavior and properties of such systems in the context of their charge properties and their ability to regulate fluid flow.”
Dr. Siddhartha Das
Finally, the team demonstrated the unexpected results of ramping up the magnitude of the electric field: the PE molecules attached to the nanochannel become deformed, and the ions that caused the instance of overscreening start to escape from the PE layer. This causes the overscreening to stop, and also reverses the direction of flow in the channel: if it was moving left to right, for instance, it switches to right-left. “No one predicted this,” Das said.
The findings are significant, Das said, because much of the interest in nanochannels relates from their ability to transport molecules. “Since flow is so important, a new discovery in this area allows us to build on our understanding of how nanochannels work and what we can do with them,” Das said. “There are other methods of reversing flow, but until now it was not known that we can accomplish this by increasing field strength.”
Published April 21, 2021