News Story
Novel Microscopy Technique to Shed New Light on Study of Cell Properties, Disease Pathogenesis
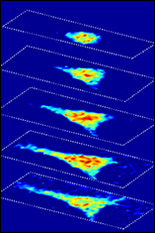
Fischell Department of Bioengineering (BioE) Assistant Professor Giuliano Scarcelli and a team of researchers from Massachusetts Institute of Technology (MIT) and Massachusetts General Hospital have developed a label-free, optical microscopy technique capable of shedding new light on how the mechanical properties of cells change in the course of aging, injury healing, and disease pathogenesis.
The technique offers promise that one day researchers will be able to identify a more exact starting point for the development of cancers, coronary disease, or even osteoporosis.
“Using light, we can measure cells inside tissue and we can look inside the cells to distinguish, for example, the properties of the nucleus and cytoplasm,” Scarcelli said.
In order to better understand how such diseases develop – and in efforts to learn more about factors that influence everyday biological functions – researchers need to get a clearer look at how properties of a cell change over time.
Every cell contains a cytoplasm – the thick solution enclosed within the cell by the cell membrane. Primarily composed of water, salts, and proteins, the cytoplasm is where most cellular activities occur. Even more, the cytoplasm serves as a means of transport for genetic material and acts as a buffer, protecting the cell’s genetic material from damage due to movement or collision with other cells.
For years, researchers have known that the interaction between the liquid and solid phases within the cytoplasm plays a prominent role in how cells deform and move. As such, the ability to map the hydro-mechanical properties of cells – such as viscoelasticity and compressibility – is critical to advancing understanding of how cell properties change as a symptom of disease in the body or as part of normal biological functions, such as when wounds heal.
Traditionally, techniques used to study the mechanical properties of cells have either required contact with cells or have produced images with limited resolution. As a result, information on the biomechanical properties of cells in 3-D environments is lacking.
“Gold-standard techniques require contact, therefore they are inherently limited to providing global averages of cell properties and to experimental situations where you have physical access to the cell,” Scarcelli said.
To address this, Scarcelli and six researchers have introduced a technique known as Brillouin optical cell microscopy for noninvasive, 3-D mapping of intracellular and extracellular hydro-mechanical properties. Their technique – published this week in Nature Methods – employs Brillouin light scattering, a process that occurs when light interacts with density fluctuations in a medium. These spontaneous fluctuations are driven by collective acoustic vibrational modes, known as phonons, in the gigahertz frequency range. In this way, Brillouin microscopy yields invaluable information on the viscoelastic characteristics of cells – and does so at a microscopic resolution no other technique can match.
As such, Brillouin microscopy opens up new research avenues for the biomechanical investigation of cells and their microenvironment in 3-D at subcellular resolution.
Continuing in collaboration with Dr. Roger Kamm at MIT, the research team is now taking advantage of the unique capabilities of Brillouin microscopy to look at a crucial property of metastatic cells – their ability to modulate their internal mechanical properties to enter and exit blood vessels to colonize distant sites. The team’s efforts recently earned them a five-year, $3 million National Institutes of Health grant to study tumor cell extravasation.
Along with Scarcelli and Kamm, Dr. William J. Polacheck, Dr. Hadi T. Nia, and Dr. Alan J. Grodzinsky of MIT, Kripa Patel of Massachusetts General Hospital’s Wellman Center for Photomedicine, and Dr. Seok-Hyun Yun of the Wellman Center and Harvard Medical School co-authored the Nature Methods paper titled, “Noncontact three-dimensional mapping of intracellular hydro-mechanical properties by Brillouin microscopy.”
With the Fischell Department of Bioengineering, Scarcelli specializes in biophotonics with strong emphasis on optical sciences and technology development. Prior to joining the department, he served as an instructor with the Harvard Medical School and Wellman Center for Photomedicine at Massachusetts General Hospital. He is the inventor in four patents, all licensed to industry, and his work with Brillouin microscopy earned him the Tosteson Postdoctoral Fellowship Award, a NIH K25 Career Development Award, and a Young Investigator Award from the Human Frontier Science Program.
The full Nature Methods is available online via http://go.umd.edu/Brillouin-microscopy
Published October 5, 2015









