News Story
BIOE Capstone Class Virtually Presents 24 Novel Projects
Despite the transition online, the Fischell Department of Bioengineering (BIOE) Senior Capstone class presented 24 novel concepts via video, tying the department's record for most projects exhibited in the annual competition. The award winners were announced on Thursday, May 21, via an letter from BIOE department chair John Fisher, and a department-wide social media campaign.
This year's projects ranged from 3D-printed modular bioreactors for skeletal tissue engineering, to a technique for gait monitoring in children with cerebral palsy.
Created and sponsored by Mrs. Susan Fischell, wife of A. James Clark School benefactor Dr. Robert E. Fischell, the Capstone Design Competition marks the celebratory end of the academic year.
This year's judges included:
- The Fischell Department of Bioengineering Advisory Board
- Fischell Department of Bioengineering Faculty
- MPowering the State representatives
The Fischell Department of Bioengineering receives support for its Capstone Design competition from the Fischell Family, the University of Maryland MPowering the State strategic partnership, and industrial sponsors AgNovos, and Becton, Dickinson and Company. Additionally, Capstone students often receive mentorship support from industry representatives and/or clinicians, including investigators from the University of Maryland School of Medicine, Children's National Health Center, the U.S. Food and Drug Administration, the National Institutes of Health, and the University of Maryland Robert E. Fischell Institute for Biomedical Devices.
 Team 1: Designing a Novel Core Temperature Cooling Device to More Effectively Treat Hyperthermia and Heat Stroke
Team 1: Designing a Novel Core Temperature Cooling Device to More Effectively Treat Hyperthermia and Heat Stroke
Noah Ashley, Benjamin Douty, Brenna Hohl, Peter Marx, Aliyah Taule
Advisors: Dr. Shawn He (BIOE Professor); Dr. Josh King and Dr. Alison Grazioli (University of Maryland School of Medicine)
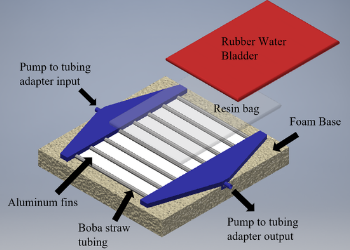
Team 1’s final Capstone project focused on designing, developing, and testing a novel core temperature cooling device to more effectively treat heat stroke and hyperthermia. Currently there is a strong unmet need for a rapidly deployable, portable, and effective method to treat patients that present with heat stroke symptoms. If left untreated, heat stroke results in organ failure and even death. The device proposed through this project is a conductive cooling device that the patient will lay on top of. As cold water is pumped through the channels of this device, the flow of the ice water will promote rapid heat transfer from the patient to a conductive resin, galinstan, to the water flowing through the system. The resin bag filled with conductive galinstan allows for maximum skin surface area to be targeted while still allowing for normal standard of care practices, specifically defibrillation and sterile central line insertion, to take place. Additionally, the device includes various electronic sensors, such as temperature sensors and LEDs that will assist the clinician with proper device operation. A prototype consisting of one cooling unit module was constructed. MATLAB modeling, Fusion 360 simulations and Arduino simulations were used to verify the success of the prototype.
 Team 2: Novel Magnetically Actuated Pill Camera for Evaluation of Bladder Function
Team 2: Novel Magnetically Actuated Pill Camera for Evaluation of Bladder Function
Hanna Briskin, Yahya Cheema, Asher Kim, Emma Moore, Pavan Vemulakonda
Advisors: Dr. Giuliano Scarcelli (BIOE Assistant Professor), Dr. Axel Krieger (Department of Mechanical Engineering/Robert E. Fischell Institute for Biomedical Devices), Dr. Michael H. Hsieh (Children's National Medical Center), Dr. Lamar Mair (Weinberg Medical Physics)
Urodynamics includes a series of tests to evaluate bladder function. Current methods of bladder evaluation involve catheterization which can cause pain to patients and lead to inaccuracies in measurements. The goal of this project is to produce a device that can measure volume in the bladder over a patient’s voiding cycle without causing significant pain. A half-octogonal electromagnet array was designed to anchor a pillcam at the center of the bladder. The array consists of eight electromagnetic patches with 130 turns per patch. In the final design, four RoboClaw motor controllers supply current to tightly wound coils on each electromagnetic patch to produce a magnetic field. In the final prototype, Team 2 3D-printed electromagnetic patches and demonstrated the construction of a patch by coiling four layers of 13 AWG copper magnet wire. In order to validate the efficacy of the proposed electromagnet array with 130 turns per patch, Team 2 conducted a combined force quantitative analysis and accounted for drag force, buoyancy force, gravity, and applied magnetic force acting on a PillCam TM COLON 2 in the expected orientation in the bladder. After calculating each force and the approximate velocity of urine in the bladder, Team 2 concluded that the half-octogonal electromagnet array can supply the required amount of magnetic force to anchor the pill at a central position in the bladder. The final product (for use in clinics) will be an adjustable waistband that contains electromagnetic patches for magnetic actuation of a miniature pillcam with associated pressure sensor. The product will have largely beneficial consequences for patients by enhancing their experience during bladder evaluations. The use of a pill camera will enable doctors and researchers to visualize bladder features and gain new insights about the presentation of bladder diseases.
 Team 3: 3D-Printed Adjustable Cast for Children with Clubfoot
Team 3: 3D-Printed Adjustable Cast for Children with Clubfoot
Niall Cope, Maxwell Hakun, Connor Hall, John Fitzell, Chad Simon
Advisors: Dr. Kim Stroka (BIOE Assistant Professor); Dr. Anuradha Dayal, Tyler Salvador (Children's National Hospital)
ADVISORY BOARD AWARD FOR TRANSLATIONAL DESIGN
As of 2016, one in every 1,000 babies are born with clubfoot, a condition in which a child’s foot or feet are rotated at the ankle and midfoot, which requires a set of corrective casts to remedy. This means that roughly 200,000 babies are born with clubfoot each year, totaling 2 million children affected worldwide. If not treated early along in the infant’s development, this impairment can lead to an inability to walk and can require surgery to fully repair. Current methodologies for early treatment include the Ponseti or French Method with the Ponseti Method preferred in over 95% of cases. The Ponseti Method involves the development of weekly sets of casting to manipulate, shape, and correct the clubfoot condition. This methodology does possess downsides such as difficulty in keeping the cast clean and the added time to remove and remake the full cast with the new corrective element. The objective of this project is to develop and analyze a 3D printing casting method to create a waterproof and adjustable cast to accommodate patient growth and ankle movements. This design must be able to accommodate the growth of leg in the thigh and calf elements, while also allowing for modular foot element attachments at the ankle to carry out the Ponseti Method. Along with this design element, other factors such as material analysis, patient comfort, universality of design, cost, and assembly complexity must be taken into account to deliver a marketable product. The final design developed by the team contains all of the necessary design criteria set in place by the user needs, while also maintaining its core function as a treatment method for clubfoot correction. The team was able to verify the performance of the design virtually by running drop test simulations in SolidWorks and physically through tension and compression testing on earlier iterations of parts. Additionally, the team was able to assemble the final design of the cast fitted to a silicone baby leg model. Future work for this project will involve working more closely with patients to validate and further iterate on the design, as well as pursuing patenting and FDA approval, once necessary.
 Team 4: Improving Spinal Tap Interventional Procedures Using Augmented Reality and Ultrasound
Team 4: Improving Spinal Tap Interventional Procedures Using Augmented Reality and Ultrasound
Kunal Dharmadhikari, Jackson Fife, Rebecca Hirshon, Neal Kewalramani, Arun Maran
Advisors: Dr. Li-Qun Zhang (BIOE Professor); Dr. Cha-Min Tang, Dr. Paul Bigeleisen (University of Maryland School of Medicine)
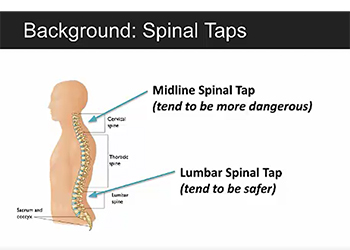
The aim of this project is to create an augmented reality program to assist in spinal tap procedures to help reduce mistakes made by newer doctors. The program will include a virtual axis that can be aligned with the patient’s spine to outline the proper trajectory for the needle during the procedure. Through quantitative analysis, it has been demonstrated that the Hololens can accurately resolve the trajectory as well as accurately rotate the axis in the 3D environment and thus can be used at the platform for this project. The team has begun prototyping of the program, including coding of the required functionality.
 Team 5: Self Expanding Vascular Cannula for Pediatric Extracorporeal Life Support
Team 5: Self Expanding Vascular Cannula for Pediatric Extracorporeal Life Support
Robert Hawkins, Collin Keating, Kurt Kunkel, Nicholas Ranallo, Ahron Verschleisser
Advisors: Dr. Zhongjun Wu (BIOE Research Professor), Dr. Karthik Ramakrishnan
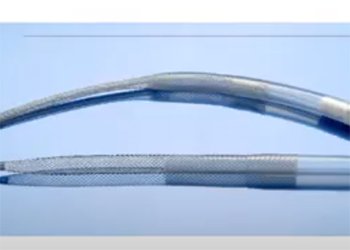
When pediatric patients in the cardiac intensive care unit (ICU) experience lung or heart failure, doctors introduce the use of a life-support procedure known as extracorporeal membrane oxygenation (ECMO). This procedure calls for a “venous” cannula to be placed in the inferior vena cava (IVC), which removes the blood from a patient. A return, ‘arterial’ cannula is placed in the aorta which returns blood to the patient after it has been oxygenated by an artificial lung. Currently, at Children’s National Hospital, cannulation for neonatal patients is achieved by surgically exposing the vena cava and aorta, through which cannulas are inserted. Time is of the essence for patients requiring ECMO with patients often requiring chest compressions until ECMO can be established. However, the chest compressions cannot be performed during the surgical insertion of the cannulas, which requires the surgery and the compressions to be alternated, leading to the cannulation surgery taking 20-30 minutes to perform. The proposed solution of a self-expanding cannula, delivered percutaneously, will address these problems by allowing faster deployment and eliminating the need for direct vessel access in the chest. Successful design and implementation of this device has the opportunity to reduce time and invasiveness of the cannulation procedure. This device will improve the efficiency of care received by pediatric patients in the cardiac ICU, and to lead to an overall increase in the survival rate of ECMO procedures.
 Team 6: 3D-Printed Microfluidic Chip for Quantifying Acquired von Willebrand Disease
Team 6: 3D-Printed Microfluidic Chip for Quantifying Acquired von Willebrand Disease
Skyler Defrank, Erin Langille, Simone Oliver, Erin Reilly, Alexi Rodriguez
Advisors: Dr. Lan Ma (BIOE Lecturer); Dr. Michael Mazzeffi, Dr. Kenichi Tanaka
As many as 3.2 million people in the United States are diagnosed with von Willebrand Disease (vWD). Type 2A vWD is a non-inherited bleeding disorder caused by high shear stress within the blood vessels, induced by mechanical circulatory support. Abnormal shear stress on the blood from these mechanical circulatory support devices causes VonWillebrand Factor (VWF), a blood clotting protein, to unfold and deactivate thus inhibiting platelets to clot at injury sites. A patient can experience various levels of protein unfolding, contributing to varying severities of acquired type 2A vWD (Ogiwara 2012). Patients with acquired type 2A vWD can experience uncontrollable bleeding, chronic inflammation, and induced anemia which can prove fatal. Increased need for mechanical circulatory support (i.e. LVAD, ECMO) as a bridge-to-transplant or cardiac support increases the prevalence of type 2A vWD; therefore, understanding the severity of VWF breakdown and coinciding blood coagulation is essential for doctors to alter a patient’s treatment plan and medication. The current market lacks a rapid, point-of-care blood coagulation test to assess a patient’s blood clotting ability due to type 2A vWD. This project verifies the current market need and proposes a microfluidic assay to detect the severity of vWD. The Microfluidic Electrical Impedance Detector (MEID) would be a non-invasive method for cardiac doctors in the operating room and intensive care units to rapidly detect the ability of a patient’s blood to clot due to the amount of VWF in their blood. The device consists of a 3D printed microfluidic chip that is supplied blood via an automated syringe pump and uses electrical impedance to detect the viscosity of blood. Viscosity of blood directly correlates to the ability of the blood to clot and thus vWD severity. To further activate clotting, the MEID mixes vWF activators (Ristocetin and Cytochalasin D) with the blood upon injection into the device and contains portions lined with collagen. The curved geometry of the microfluidic channels mixes all of the components and induces the necessary 5000 Hz shear rate to activate blood clotting. With clear, quantitative results, doctors can easily adjust patients’ treatment accordingly, improving outcomes for those on mechanical circulatory support.
 Team 7: Development of a Device that Utilizes Intermittent Compression and Cooling for Heat-Related Illnesses
Team 7: Development of a Device that Utilizes Intermittent Compression and Cooling for Heat-Related Illnesses
Mariama Barrie, Ismael Guere, Akhil Katuri, Jinan Oubaid, Enna Wu, Efrem Admassu
Advisors: Dr. Katharina Maisel (BIOE Assistant Professor); Dr. Josh King, Dr. Alison Grazioli (University of Maryland School of Medicine)
Heat stroke occurs when body temperature reaches or goes above 105°F (40.5°C). These dangerously high temperatures can cause severe complications like multiorgan dysfunction and possibly death. Current methods of treating heat stroke involve convective cooling and ice water convection. However, the standards for treating heat stroke are not efficient because the methods are limited to the resources available and/or other factors that impact the need for patient access in the treatment process. The team has designed a wearable cooling vest that uses the Peltier effect to rapidly reduce the core body temperature of heat stroke patients. From the prototyping results, the peltier devices and heat sinks were able to achieve the lowest temperature of 38.9°F with 12 volts of 2 amps. Using CAD simulations, the team has determined that our design can safely and effectively reduce the body temperature of a simulated heat stroke patient from 120°F to 99.61°F. Therefore, the peltier devices can serve as an alternative for the treatment of heat stroke patients with affective cooling abilities to be reached. With the improvements to the design, our device may serve as a viable option for treating heat stroke in the medical field.
 Team 8: Measurement of Pediatric Nasal Air Temperature with Non-Invasive Novel Temperature-Based Device
Team 8: Measurement of Pediatric Nasal Air Temperature with Non-Invasive Novel Temperature-Based Device
Viviana Bentley, Sanyukta Deshmukh, Celia Fernandez Brillet, Nidhi Kalaria, Kelsy Song
Advisors: Dr. Giuliano Scarcelli (BIOE Assistant Professor); Dr. Amal Isaiah
BEST VIDEO PRESENTATION (TIE)
Each year, approximately 500,000 children in the United States undergo tonsillectomy and/or adenoidectomy surgeries, yet only 50,000 of those children undergo polysomnography (PSG) sleep studies prior to surgery. The purpose of PSG is to diagnose Obstructive Sleep Apnea Syndrome (OSAS). Currently, this test is carried out at hospitals and comes at a high price. During PSG, several sensors are placed on the child’s body to measure different sleep parameters. However, previous studies have shown that PSG could be simplified to measurement of only four essential parameters: nasal airflow temperature, actigraphy, heart rate variability, and pulse oximetry. Most of these measurements can be easily adapted to an at home setting except nasal airflow temperature, which comes with some complications such as great discomfort, bulky signal readers, and wired connections. The discomfort of the nasal sensor for pediatric populations as well as its invasive nature prevents its translation to an at home setting. For this reason, the purpose of this project is to redesign the pediatric nasal airflow sensor such that it is non-invasive (no nasal insertion), wireless (does not require long cables attached to it), inexpensive, user friendly (could be used in an at home setting), while at the same time preserving diagnostic accuracy. In the proposed model herein, an optimized version of a small digital temperature sensor was achieved by shaving off some of its casing, thus improving fast rate detection. Then, an appropriate controller board with wifi capabilities was coupled with the sensor along with a high speed online reading platform. The optimal material to be used to house the sensor was determined to be ethylene-propylene via comparison using finite element analysis. Finally, a smartphone app was developed using the device specifications and a friendly user interface targeted to the patient's parents. As a result, the final assembly is able to record raw inhalation and exhalation temperatures that are displayed as a high resolution cyclic pattern in a smartphone app. Ultimately, this de novo model will have a significant impact by facilitating the implementation of PSG in an at-home setting, bringing the costs down, decreasing complexity, and most importantly, improving and increasing OSAS diagnosis among children in the United States.
 Team 9: Guidance Device for LVAD Placement
Team 9: Guidance Device for LVAD Placement
Lauren Moyer, Anushka Gerald, Briana Ho, Rachel Prucnal, Sabrina Martin
Advisors: Dr. Alisa Clyne (BIOE Associate Professor), Dr. Joseph Rabin (University of Maryland School of Medicine)
Patients with heart failure occasionally require a ventricular assist device in order to pump blood from the left ventricle into the aorta. During the implantation surgery, a cannula is inserted into the left ventricle, onto which the pump is attached. If this cannula is not within seven degrees in line with the mitral valve, the blood flow may become turbulent, potentially leading to thrombosis and device failure. Currently, surgeons use transesophageal echocardiography imaging and simple touch during surgery to choose the position of the cannula. Surgeons first attach a sewing ring to their chosen position, without knowing the true location of the mitral valve. Once surgeons attach the sewing ring and core the heart, they are unable to change the position of the cannula, with the exception of tying the drive line to a rib. Therefore, this process is fairly subjective and prone to error since the surgeons do not have a way to align the cannula with the mitral valve. To address this problem of cannula alignment with the mitral valve, Team 9 proposes a sewing ring attachment that would be able to rotate and lock the cannula in the optimal position. The attachment uses a bearing and socket mechanism with a ring lock on the bearing and a locking mechanism in the base. This would allow the surgeon to loosen the screw in the locking mechanism on the base, rotate the cannula to align with the mitral valve, and relock the cannula and bearing in place. This design was the result of a long and iterative design process which involved other design ideas such as a curvature finder device, augmented reality, ultrasound, and others. Overall, Team 9 decided on a sewing ring alteration due to its potential accuracy, integration into surgeon workflow, and feasibility to produce. The team iterated the sewing ring alteration designs and clamping mechanisms as well and decided to continue testing with the “onion” design as it had the most angulation and best clamping strength.
 Team 10: Adverse Environmental Stimuli in the ICU
Team 10: Adverse Environmental Stimuli in the ICU
Ainul Abdul Rahim, Mauricio Defngin, Stephen Frocke, Erin Lee, Faith Olulana, Maddie Curtis
Advisors: Silvina Matysiak (BIOE Associate Professor), Dr. Jeffrey Hasday (University of Maryland School of Medicine)
MPOWER AWARD
The Intensive Care Unit (ICU) is a high-stress environment that contains adverse stimuli such as bright lights and high levels of noise. These adverse environmental stimuli contribute to a high prevalence of sleep deprivation in the ICU, negatively impacting the body and its natural circadian rhythms. Sleep deprivation can impair the body’s immune system, which can leave ICU patients even more susceptible to infections, disease, and may affect patients' ability to heal. To address this clinical issue, the development of a localized environment prototype is proposed, with the ultimate goal to aid patients in retaining a regular sleep cycle in the evening to night hours, when doctors and nurses encourage proper and sufficient rest. The design concept is a hood-like structure that filters blue light, actively cancels noise (ANC) and will allow patients to rest comfortably without impeding necessary ICU workflow.
 Team 11: Chest Tube Guiding Device
Team 11: Chest Tube Guiding Device
Mariam Adwan, Christopher Bach, Khazar Choudhry, Ray Rada, Jordyn Walker, Lauren Christensen
Advisors: Dr. Lex Schultheis (BIOE Research Professor; Fischell Institute), Dr. Joseph Rabin (University of Maryland School of Medicine)
The goal of this project was to improve patient safety during thoracostomy procedures by designing a device to reduce muscle and tissue trauma. Before starting the designing process, all the forces involved in a thoracostomy procedure needed to be characterized so tolerances for the device materials and different potential designs could be established. Since these forces weren’t found in any available resources, they were measured through experimentations using various tools and test mediums. These measured forces helped to design a device that uses a dilation mechanism to decrease muscle trauma and prevent internal bleeding. In the future, the device will be further prototyped according to the input from both the faculty and clinical advisors. Lastly, the finalized and thoroughly tested device design will be submitted to the FDA for a presubmission and eventually approval.
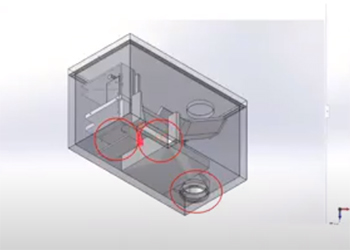 Team 12: Automated Sharps and Procedural Instruments Divider
Team 12: Automated Sharps and Procedural Instruments Divider
Jules Allbritton-King, Haley Bertrand, Kay Htut, Sophie Lyle, Raymond South, Khalil Chughtai
Advisors: Dr. Helim Aranda-Espinoza (BIOE Associate Professor), Dr. Kenneth McKinley (Children's National Hospital)
1ST PLACE
Reprocessing and reuse of procedural instruments and surgical tools has been shown to be safe for patients, financially beneficial for hospitals, and environmentally sustainable. The Association of Medical Device Reprocessors (AMDR) currently engages thousands of hospitals in proper sorting and resterilization procedures for medical devices, however, many hospitals are unable to participate in medical device reprocessing due to a lack of infrastructure and manpower required to sort through discarded medical waste and retrain hospital staff. The purpose of this Capstone design project is to develop an automated device that will sort discarded medical instruments based on their potential reprocessibility, thereby removing the need for costly retraining or hiring of additional staff members. The proposed device will be able to distinguish between reprocessible and non-reprocessible medical instruments on the basis of the item’s physical properties. The instruments deemed reusable will be sorted into a separate receptacle to be sent to reprocessing facilities. The reprocessed sharps could either be reused for in-house applications, resold at a lower price, or donated to global health efforts.
 Team 13: Detection and Visualization of Bioburden on Surgical Instruments
Team 13: Detection and Visualization of Bioburden on Surgical Instruments
Kate Balatgek, Christopher Delre, Zaafira Elham, Sarvenaz Hedayati, Nikita Tatwadhia
Advisors: Dr. Yang Tao (BIOE Professor), Dr. Jonathan Pearl (University of Maryland School of Medicine), Dr. Stephen Restaino (Maryland Development Center)
Surgical instruments undergo intensive sterilization and inspection after use to prepare them for reuse. These tools, however, are inspected manually and human error can cause instruments with bioburden to be packaged and sent into the operating room. Bioburden is any bacteria residing on the surface of an instrument. The accumulation of bioburden on an instrument during a procedure is unavoidable. Ineffective sterilization and inspection may
produce a tool with contaminants. A single surgical instrument retaining bioburden results in increased patient and hospital costs, as well as delayed surgical procedures, but most importantly, it causes an increased risk of surgical site infections during surgery. This project implements a three-part design: the first component is a chemical soak, in which used surgical instruments are manually soaked in fluorescamine and acetone. Fluorescamine is a fluorescent dye that only binds to bacterial proteins, so wherever there is bioburden, there is a protein that fluorescamine can bind to. The second component is a viewing box in which the user can manipulate and clean instruments of various sizes. The box contains an ultraviolet light to excite fluorophores in fluorescamine and a UV light filtered webcam that takes images of emitted blue light. The final component of the design is an imaging software that detects and alerts the user of the presence and location of bioburden. This design aims to provide sterilization inspectors with a reliable and efficient method for detecting bioburden, thus reducing the amount of bioburden that persists on instruments after the sterilization process. A visualization and detection system that reduces the number of bioburden related events in the hospital will allow patients to feel safer and staff to feel confident in the utilization of surgical instruments. The results found the ideal concentration of fluorescamine to be 0.6 mg/ml. We were also able to develop a successful imaging program that can detect and visualize bioburden with a true positive rate of 96% and a false positive rate of 13%. Future work for this design includes building the final physical product and possibly automating this process further to make it more time-efficient.
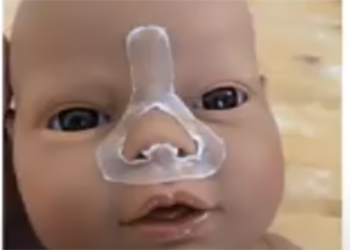 Team 14: Protective Barrier for NICU Patients on CPAP
Team 14: Protective Barrier for NICU Patients on CPAP
Richa Gupta, Lily Ha, Jackson Lange, Anna Latzko, Nora Moore
Advisors: Dr. William E. Bentley (BIOE Professor; Fischell Institute), Lawrence Sims (Emory University Hospital)
Continuous Positive Airway Pressure, or CPAP is a vital treatment utilized for premature infants in the Neonatal Intensive Care Unit (NICU) with underdeveloped lungs and airways, offering a direct oxygen supply and pressure for respiratory support. Despite its widespread use, a major downfall in the design of CPAP is the point of contact between the mask and premature infants facial skin, as the pressure and friction from the mask commonly lead to irritation, pressure ulcers, and deformities. This proposal outlines the design of a protective barrier between the mask and premature infants skin. This proposed barrier is designed to cover the pressure points specific to the facial topography of a premature infant. In addition, a detailed investigation of the hydrocolloid composition of this design, with optimal combination of polyisobutylene, sodium carboxymethylcellulose, gelatin and pectin, would present material advantages to prevent adverse reactions with the skin while maintaining desirable physical and functional properties. The outcome of this project was only a prototype. The size and shape were finalized, but the material was only synthesized once. To compensate for the material shortcomings, a quantitative analysis was conducted to provide insight to our design. This progress provides great potential for a novel barrier that will enhance the efficacy of treatment while minimizing health complications for premature infant CPAP.
 Team 15: Gait Monitoring in Children with Cerebral Palsy
Team 15: Gait Monitoring in Children with Cerebral Palsy
Quinton Burke, Roybn Collins, Patrick Haggerty, Valeria Kaufman, Sharon Puthumana
Advisors: Dr. Ian White (BIOE Associate Professor), Dr. Paola Pergami (Children's National Hospital)
Cerebral palsy (CP) is a group of neurological disorders that can affect an individual’s movement and posture. While the symptoms can vary, CP patients' problems can range from mild, in which the patient might walk awkwardly but will require no further assistance, to severe, in which the patient cannot walk without assistance or at all. Two of the main issues with patient gait is incomplete knee extension and lack of heel-to-toe movement while walking that results in crouched gait. Physical therapy has been a proven mechanism in improving a patient’s gait, but the costs and burdens associated with physical therapy do not make it a feasible option for all. Therefore, the lack of continuous patient implementation of physical therapy exercises in the home and at the therapist’s/doctor’s office results in stagnation or even regression of patient gait. Thus, bioengineering students from the Capstone Class of 2019 began working on two designs for a take-home approach to physical therapy. One group focused on measuring how CP patients were walking on their feet through the “pressure sole,” a sensor to output data from the toe, ball, heel front, and heel back. Another group focused on measuring knee flexion/extension through angular data with the “knee extension monitoring” sensor. This year, Team 15’s project aimed to develop a collaborative and user-friendly approach to measuring and improving gait in CP patients. By using data gathered by last year’s Capstone class, this year’s project worked towards combining knee extension and heel movement monitoring into one device. Prior to the impacts of COVID-19, a major aspect of our project was to design an Android app to analyze CP patient walking patterns to provide motivation for better walking habits outside of physical therapy. By the end of the academic year, we were able, however, to complete the design of two separate systems with their own sensors and recording methods. For the knee component, we were able to accurately measure knee angles between 0 and 70° within a few degrees which is the necessary range for normal walking patterns. For the foot component, the group was able to measure output voltage as a function of stepping force, where voltage had a direct relationship to pressure. The group was able to couple these systems as well through the implementation of a microcontroller that could simultaneously transmit data from both devices to a generic user interface via Bluetooth. The generic user interface was able to analyze data and provide preliminary classifications of complete knee extension and heel-to-toe movement for users. The app portion of the project was able to display different tabs such as practice, statistics, and games, but was not yet able to communicate with the microcontroller via Bluetooth to receive data. Through each of these tabs, the group aimed to help encourage and motivate patients to successfully complete their exercises at home as well as to properly inform parents and physicians of patient progress.
 Team 16: Calibration Device for BD Diagnostics Cor Sample Creator of DNA-based HPV Testing
Team 16: Calibration Device for BD Diagnostics Cor Sample Creator of DNA-based HPV Testing
Ayah Arafat, Michael Hildreth, Maeesha Noshin, Julia Pinsky, Saya Usui
Advisors: Dr. Ian White (BIOE Associate Professor); Leon Tate, Chris Tesluk (BD Diagnostics)
BD has designed The BD Cor system to run a number of assays on a single sample. It generates results regarding women’s health, sexually transmitted infections, and the gastrointestinal system. This system generates up to nine different DNA-based assays by processing a small patient sample into a tube and adding one of four different diluents. The diluent contains buffers which prepare the sample for DNA amplification. Our team has been tasked with the enhancement of the initial calibration of the diluent pump by decreasing the window of calibration from 16% to 3%. Currently, the pump is manually calibrated to an accuracy of +/- 8 % and installed in a time-consuming and inefficient fashion. On average it takes an individual one hour to calibrate each pump and there are 8 pumps total in the BD Cor system, totalling an entire work day. We are hoping to increase the accuracy of volume delivery by decreasing the window of calibration to +2/-1 %. This will prolong the life-expectancy of the pump because if the pump is more accurately calibrated, it will allow the pump more time to drift out of the optimized window as it works in the machine due to wear. The improvement in accuracy will also improve upon the overall efficiency of the system with a new, more advanced calibration approach for the diluent pump. The final design was decided upon using a pugh matrix comparing four different designs. Although we were unable to complete a final device, our concept and model prototype was complete. The product was meant to calibrate the pump using a motor to turn the screw controlling the flow output of the pump. The motor would have been controlled by an arduino microcontroller and the arduino would have received input from a mass scale that was plugged into a computer. We took various steps to simulate the final product in order to know what dimensions it needs to be and how to code the processes. The first step was to find a trend between the volume output of the screw and degrees of rotation in order to create a formula which can be used to efficiently and quickly calibrate the pump. Before the prototype was assembled, the code was developed and tested using a circuit with LED lights to mimic the pumps and motor and a potentiometer to represent the mass scale. The code developed for this would have been only slightly tweaked to be applied to the end product and incorporate the mass scale. The project should have been completed in 8 months with a budget of under $600.
 Team 17: Pediatric Jaw Thrust Device
Team 17: Pediatric Jaw Thrust Device
Mitchell Carlson, Diego Diaz, Jordan Groff, Marie Hatch, Hayat Jabbour
Advisors: Dr. Gregg Duncan (BIOE Assistant Professor); Dr. Brad Ralston, Dr. Richard Kaplan (Children's National Hospital)
Upper airway obstruction is a common problem during and after surgical procedures that require anesthesia. It is caused by the tongue obstructing the airway of a patient, hindering their ability to breathe. Common symptoms of upper airway obstruction include drooling, swelling of the face and tongue, difficulty breathing, wheezing, unusual breath sounds, choking, unconsciousness, and more. To combat this, the jaw thrust maneuver is done by medical professionals. This mechanism entails pushing the patients head up, lifting their jaw on both sides, and opening their mouth in order to prevent any upper airway obstruction.
Medical professionals must remain at the patient’s bedside to perform this maneuver, and oftentimes also must hold the anesthesia mask in place. This causes pain in clinician’s hands, a shortage in available clinicians, and diverges attention away from other complications. Due to this, several jaw thrust devices have been created. These attempts to replace the maneuver by completing the mechanism using extendable arms and a force exerted on the patient. Some devices have been effective, however all of the reviewed devices are either extremely expensive, not suitable for newborns/children, and/or are very invasive.
Team 17's goal for this innovation is to create a device that will replace the medical professional completing the jaw thrust task and instead simply allow the device to be placed easily and quickly for pediatric patients. The team's device includes a placement platform for the patients head and neck as well as extendable arms that function along a stackable base, using a spring-loaded mechanism to push the patient’s jaw upright and contracting back into a stackable encasing. This allows for easy storage and simple set up. Our device will also include a detachable mask component to address the issue of clinicians’ hands cramping due to the constant holding of the anesthesia mask. The total cost of this proposed device is currently $215.23.
 Team 18: Reusable Autoclave Tester for Austere Environment
Team 18: Reusable Autoclave Tester for Austere Environment
Christopher Beekey, Robin Brocato, Aviva Borison, Jillian Stabile, Rachel Willis
Advisors: Dr. Steven Jay (BIOE Associate Professor), Kevin Aroom (Fischell Institute)
The primary focus of this project is to develop a robust system that will validate the conditions of an autoclave to ensure that it reaches those required for adequate sterilization of medical equipment. Though the prior has previously been accomplished in medical settings with ample resources, the same cannot be said for those in austere environments with strict limitations. The requirements for this device will be to assess the ability of an autoclave to reach the desired temperature and pressure to ensure sterilization. To achieve this, the device is split into two areas for specific focus on both pressure and temperature. While aiming to meet these requirements, the device to be designed must also adhere to tight constraints. These constraints include, but are not limited to, repeated use over 200 cycles, no use of electronics or battery power, and simplistic nature wherein any personnel could be easily trained on how to properly implement and gather results. An instruction manual will be generated that does not utilize written language for ease of use. The manual will contain numerical unit conversations for easy translation within different unit systems. Over the course of the semester, Team 18 was able to successfully fabricate the temperature detector and progress with testing. The fabrication of the pressure detector was started and further modeling was achieved for proof of concept of the device. The user manual was developed for both the temperature and pressure detectors. Team 18 successfully demonstrated the ability for our device to translate and achieve original goals.
 Team 19: Disposable Delivery Device to Automate Weight-Based Dosing Utilizing Bristojet Packaged Emergency Drugs
Team 19: Disposable Delivery Device to Automate Weight-Based Dosing Utilizing Bristojet Packaged Emergency Drugs
Carly Boden, Arafat Fasuyi, Aida Kebede, Zachery Keepers, Olivia Wolcott
Advisors: Dr. Angela Jones (BIOE Senior Lecturer), Dr. L. K. Walker (University of Maryland Medical College)
3RD PLACE (TIE)
Additional information available soon.
 Team 20: Autoclave Verification Device for Developing Countries
Team 20: Autoclave Verification Device for Developing Countries
Madeleine Bowcutt, Austin Elko, Brianne Heverling, Miriam Janssen, Samantha Stuart
Advisors: Dr. Hubert Montas (BIOE Professor), Kevin Aroom (Fischell Institute)
3RD PLACE (TIE)
The disparity between healthcare-associated infection (HAI) rates in developed countries (4.5 incidences per 100 patients in the US), and limited resource countries (15.5 incidences per 100 patients) delineates the need for health care developments in resource limited countries. Developed countries have various measures that reduce HAIs, including sterilization of medical equipment. However, these sterilization verification methods are unsuitable for austere environments.
The objective for this project was to develop an autoclave verification device to reduce nosocomial infections by ensuring proper sterilization of medical devices in developing countries. By using a bimetallic strip, controlled flow, and a pressure diaphragm this device will verify that the autoclave reached a temperature of 121°C for at least 30 minutes under at least 15 psi. The device was designed to have easy to read instructions and simple reset to break technical and language skill barriers. Additionally, it was designed to be reusable, robust, non-electronic, and affordable, which are necessary requirements for effective medical devices in austere environments. In the future, Team 20 hopes to verify the device using thermocouples and pressure transducers, and the robustness by running the device through repeated autoclave cycles. The group hopes to continue its collaboration with Engineering World Health to send the device to hospitals and medical centers in need, and ultimately reduce HAIs in developing countries.
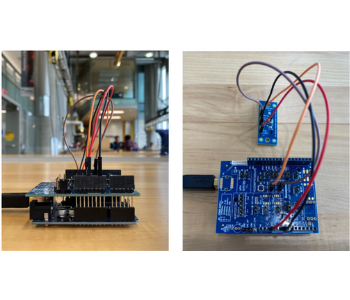 Team 21: Intraosseous Implant Site Pressure Sensing Device
Team 21: Intraosseous Implant Site Pressure Sensing Device
Robert Allen, Julia Edwards, Aleksandr Kovalyonok, Olivia White, Jennifer Xue
Advisors: Dr. Joe Huang (BIOE Assistant Professor), Dr. Jonathan Shaul
BEST VIDEO PRESENTATION (TIE)
Intraosseous pressure is one of the many indicators of bone health, with unusually high pressures signifying inflammation and tissue degradation. In the context of surgery, intraosseous pressure can serve as a gauge for patient condition alongside other somatic outputs, allowing medical professionals to detect adverse events as they occur and reduce the risk of surgery for demographics at higher risk of complications. Currently, there is no standardized method for measuring pressure directly from bone, with most alternatives relying on blood or fluid pressure data from surrounding tissue to approximate pressure values. The goal of this capstone is to develop a pressure sensor compatible with an AgNovos Healthcare bone graft kit that can transmit data to a separate monitor for real-time pressure assessment. The sensor was constructed using a Honeywell micro pressure sensor and sensor evaluation kit in conjunction with a starter Arduino board. Measurements were taken via a polyurethane tube probe affixed to the sensor and displayed through Honeywell graphing software complementary to the sensor and evaluation board. The sensor was found to successfully detect pressure changes within the proof-of-concept model in response to filling the “surgical” cavity with fluids of increasing viscosity to simulate the implant material manufactured by Agnovos. These findings encourage additional testing with bone cement, and future studies could include more anatomically-relevant models, such as a 3D-printed femur or cadaver to further validate the sensor.
 Team 22: The Caffeine for Apnea in Preterms (CAP) Patch
Team 22: The Caffeine for Apnea in Preterms (CAP) Patch
Hoag-Thien Cao, Tiffany Cao, Anna Kanu, Amarachi Ude, Sarah Young, Miles Patton
Advisors: Dr. Christopher Jewell (BIOE Associate Professor), Dr. Rose Viscardi (University of Maryland School of Medicine)
Preterm infants are born in a harsh environment, with extremely underdeveloped organ systems. In particular, their respiratory systems are especially underdeveloped, which runs the risk of impaired oxygen flow to the brain and other parts of the body. The most prevalent health issue they face is apnea, which is the cessation of breathing for more than 20 seconds. This decreases the amount of oxygen being delivered to the brain, thus exacerbating neurological and respiratory systems. Current treatments for apnea of prematurity are normally administered hours after birth, which can affect the preterm infant’s recovery time and increase the risk of intubation or ventilation. Both options are rather harmful to the infant’s underdeveloped esophageal system. Thus, the goal of our project is to implement transdermal drug administration. Transdermal drug delivery presents a promising alternative method due to its noninvasive nature and its potential for steady-state drug delivery. Apnea of prematurity can specifically be treated with caffeine; therefore, this project proposes the development of a skin-safe patch which transdermally delivers caffeine to preterm infants in a controlled, continuous manner. Our patch would administer caffeine to the infant via skin-sensitive microneedles in a steady-state fashion for at least a week or until the baby’s systems have adequately matured. Our theoretical prototype consists of dissolvable microneedles on an adhesive patch. The prototype must go through several optimization tests in order to be fully standardized. Diffusivity tests on agarose gel and hairless pig skin models will allow diffusivity and absorption data to be collected. These results will allow us to refine our design in order to ensure that the proper dosage is delivered and therapeutic levels of caffeine are maintained in the infant for at least a week. Additionally, we may look at current caffeine patches on the market as examples, these patches are used for adults and are limited to use for preterm infants. Since adult patches are made for mature skin and adult weight, they would not be of any benefit to sensitive preterm infants, who weigh a fraction less. Although our project ran into the significant barrier of lab closures due to the coronavirus pandemic, we were able to accomplish the refinement of our design, implementation of design modeling/computer simulation, and strengthening the business and ethical cases for our product. Based on several interviews with current physicians and nurses, as well as previous research, we can conclude that the potential of a transdermal drug patch is immense, with the ability to alleviate financial burden, physical discomfort, and mental stress for both the infants and parents.
 Team 23: Use of an EEG Based AI Model and GUI for Alzheimer’s Disease Screening
Team 23: Use of an EEG Based AI Model and GUI for Alzheimer’s Disease Screening
David Boegner, Christopher Look, Anoop Patel, Dhruv Patel
Advisors: Dr. Edward Eisenstein (BIOE Associate Professor), Dr. Joseph Dien (University of Maryland)
Alzheimer’s Disease is the 6th leading cause of death and the most expensive disease in the United States. While there is no effective treatment or cure for the disease, there is an urgent clinical need for an early diagnosis. An early diagnosis allows patients to better plan for their future financially and legally, begin medication earlier that may have beneficial clinical effects, rule out & manage comorbidities, and enter clinical trials for novel drugs. Alzheimer’s disease is primarily characterized by neural injury, hyperphosphorylated tau aggregation, and Amyloid Beta-42 accumulation. These neuropathological features may begin upto 10 years prior to initial clinical symptoms, and by that time, there is already irrecoverable damage done to the brain. Alzheimer’s screening is not routine because current modalities including PET and MRI are too costly, invasive, and require specialized training to be used on mass populations. This study evaluates the potential of a portable Electroencephalogram (EEG) system for screening AD, based on machine learning classifiers through Dominant Frequency (DF), Higuchi Fractal Dimension (HFD), and Fast Synchronization Likelihood (FSL) biomarkers. Through IRB approved protocols, 18 Healthy (HC) and 32 Alzheimer’s Disease patient resting state EEG recordings were observed from the CATField study from NewCastle University for analysis. Mann-Whitney U test was performed for all biomarkers extracted for 21 electrodes for all patients to determine spatial significance of electrodes by clinical group and thus inform potential pathological progression of disease. Linear regression was used to describe the correlation of DF, HFD, and FSL with clinical scores such as Mini-Mental State Examination (MMSE). Spectral slowing was observed with statistical significance up to p
 Team 24: 3D Printed Modular Bioreactors for Skeletal Tissue Engineering
Team 24: 3D Printed Modular Bioreactors for Skeletal Tissue Engineering
Dhruvesh Amin, Sally Choi, Samuel Finkel, Erik Hung, Gabriella Schito
Advisor: Dr. John Fisher (BIOE Professor)
2ND PLACE
Osteoarthritis is the most common joint disorder in the United States, affecting over 30 million people. The incidence of osteoarthritis, along with other bone disorders, will increase greatly due to factors such as age and obesity. Current treatments for bone disorders, including autologous and allogeneic bone grafts, leave much to be desired as they are expensive, involve extensive surgery, are limited in supply, and have issues with osteoinductivity. Skeletal tissue engineering is a more promising solution for achieving optimal differentiation of stem cells into osteoblasts. Team 24 created a 3D printed modular bioreactor to facilitate research conducted within this field that better mimics the in vivo environment than current technologies. The goals of the project were to (1) create a 3D printed bioreactor (2) apply an equal compressive force on scaffolds within the chamber and (3) introduce an upward flow of media at a rate of 1mL/min through the system. The design involves a main chamber with a lid, force applicator, 6 scaffolds, rack and pinion, and an inlet and outlet port for media flow. The design went through different iterations concluding at the current design which was tested computationally using SolidWorks force and flow simulations. The stress and strain results show that when a 50 N force was applied to the scaffolds, the scaffolds did not undergo failure. The flow simulation showed that the max shear stress experienced by the scaffolds from the flow is under 0.2 Pa and the velocity trajectories showed a proper dispersal of media. The group’s next step is to verify that its bioreactor system will facilitate specific cell line differentiation through controlled studies analyzing osteogenesis.
Published May 21, 2020








