News Story
Jewell Lab Awarded Two NIH R01 Bioengineering Research Grants

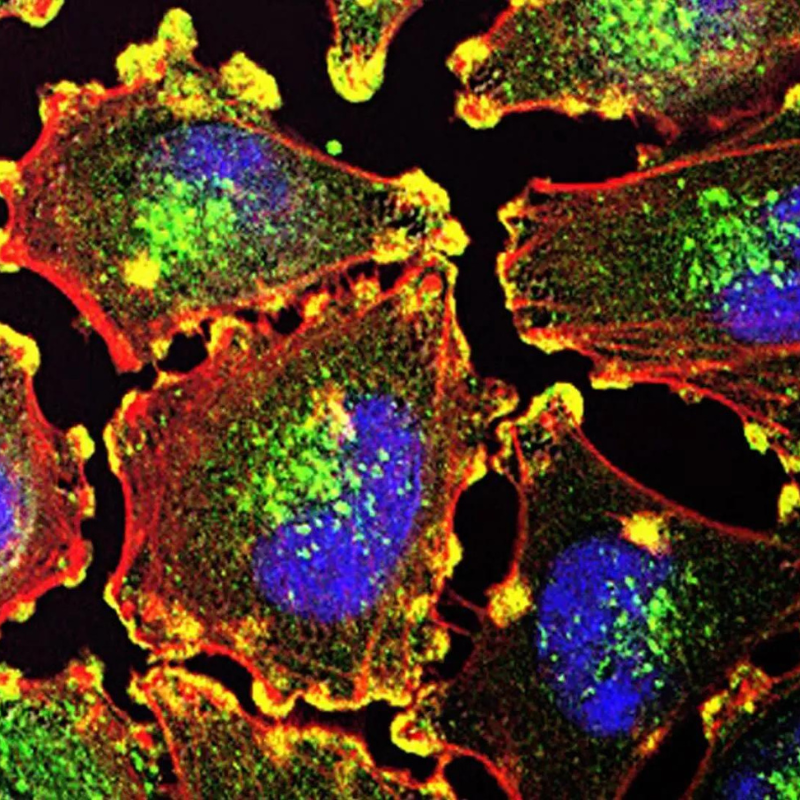
Photo by Alan P. Santos
This spring, Fischell Department of Bioengineering (BIOE) Associate Professor and Associate Chair Christopher Jewell was awarded two new National Institutes of Health (NIH) Research Project Grants (R01) to improve multiple sclerosis treatments and the design of vaccines for cancer or infectious diseases, respectively.
The grants – both of which are classified as NIH Bioengineering Research Grants (BRGs) – total $3 million in funding over the next four years. Combined with two other active NIH R01 grants for which Jewell leads or is co-funded, an ongoing Research Merit Award he leads at Baltimore's U.S. Department of Veterans Affairs, and grants from leading charitable foundations, Jewell and his Immune Engineering Lab are supported by more than $7.5 million in active research funding.
The NIH BRG program aims to develop innovative technology with the potential for a significant impact on biomedical research by integrating multidisciplinary approaches and quantitative principles.
Rational Design to Improve Vaccine Potency and Simplify Clinical Translation
Vaccines continue to play a transformative role in preventing and treating diseases ranging from infectious pathogens to cancer. These technologies work by harnessing the specificity of the immune system to clear pathogens without targeting the body’s own cells. To achieve these goals, Jewell and his lab are working to shed new light on adjuvants – molecules added to vaccines to enhance their function – and how they can be used to support design of next-generation vaccines that elicit responses for specific diseases. The team is working to combine natural biomaterials to create a simple nanotechnology platform that reveals how combination adjuvants work together.
“Biomaterials hold great potential because they offer the ability to deliver multiple cargos; however, many materials – polymer particles, for example – exhibit intrinsic features that can activate inflammatory pathways even in the absence of other immune cues,” Jewell said. “This feature can be harnessed in vaccination, but it also hinders rational design because the role of each vaccine component is clouded by the intrinsic effects of the carrier. Materials that offer features of biomaterials – such as co-delivery – but that improve the modularity and definition of vaccines could provide new knowledge of how combination adjuvants polarize immunity, and inform the design of a new generation of vaccines that elicit tunable responses.”
Toward this goal, Jewell and his team designed a new class of vaccines by creating immune polyelectrolyte multilayers (iPEMs) built entirely from immune signals. These iPEMs are self-assembled on particle templates during production. Then, the templates are dissolved to leave hollow capsules that consist only of the immune cues the lab wants to deliver, eliminating the need for carrier components.
“Nanoparticles vaccines for infectious disease and cancer have great potential, but in most cases, the polymer, lipids, or other components just serve as carriers,” Jewell said. “This causes the signals to be delivered in a less concentrated way compared with iPEMs, where 100 percent of the vaccine or therapy is the active immune signal. In other words, the adjuvants and other components serve both as cues to the immune system, as well as structural components to mimic the useful features of biomaterials, such as efficient delivery to immune cells and delivery of multiple signals.
“This platform gives us a simple way to concentrate signal delivery to the immune system, as well as the modularity to turn on specific combinations of pathways,” he continued. “Both of these features are important in effective, customized immune responses to tumors or pathogens.”
Microneedle Patches to Improve Multiple Sclerosis Treatments
Multiple sclerosis (MS) is an autoimmune disease that results in a debilitating loss of coordination and motor function. In MS, the immune system incorrectly recognizes components of the central nervous system, causing inflammation and destruction of myelin, the fatty substance that surrounds and protects nerve fibers. When this happens, nerve fibers and cells are damaged, leading to a loss of motor function and other complications.
According to the National Multiple Sclerosis Society, MS affects more than 2.5 million people worldwide.
To date, there is no cure for MS, only drugs that aim to help slow progression of the disease. Unfortunately, because many patients lose the coordination to inject these medications, patient compliance often requires in-home assistance or transportation to a clinic.
To address this challenge, Jewell is leading a team of bioengineers, immunologists, and MS-focused clinicians to develop easily-applied, degradable microneedle patches. This approach might eventually improve the effectiveness of current MS drugs, while also enabling patients to administer their own medicine – even in cases where the disease has reduced the patient’s motor control.
The group is working to create microneedles from existing MS drugs that are widely-prescribed to treat the disease. Jewell and his team will design and test these technologies in pre-clinical animal trials, and compare the efficacy of the drugs designed as microneedles to the current injectable forms. They hope their innovative technique will improve treatment compliance by allowing easy administration, while also improving efficacy by targeting specialized immune cells in the skin.
Jewell and his team are no strangers to advancing MS and autoimmune disease research. Jewell’s lab was previously supported by grants from the National Multiple Sclerosis Society, and last year was awarded another four-year, $1.4 million NIH R01 grant for efforts to study how the local environment of lymph nodes – specialized immune tissues – contribute to MS and potential new immunotherapies.
Also last year, the NIH awarded a $1.7M, four-year grant to George Washington University Cancer Center Assistant Professor of Medicine and BIOE adjunct Rohan Fernandes to advance his efforts to use nanoimmunotherapy to improve cancer treatments. Jewell is leading a part of the project centering on adjuvant delivery. The overall goal of the collaborative grant is to develop new, multi-pronged nanotherapies that can selectively destroy tumors which may previously have been untreatable.
In addition to his BIOE affiliation, Jewell is a faculty member of the Robert E. Fischell Institute for Biomedical Devices, a research biologist with the VA Maryland Healthcare System, and a full member of the University of Maryland Marlene and Stewart Greenebaum Comprehensive Cancer Center.
Published June 24, 2019